Carboxymethyl glycosaminoglycan derivative and preparation method and application thereof
A technology of polysaccharide derivatives and carboxymethylamino, which is applied in the field of carboxymethylaminopolysaccharide derivatives and their preparation, can solve the problems of restricting the application of chitosan, failing to meet market requirements, weak biological activity, etc., and meet the requirements of equipment and raw materials Easy to obtain, easy to promote, and low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In this embodiment, the target compound carboxymethyl amino polysaccharide derivative is synthesized according to the above synthetic route, specifically:
[0028] 1) Preparation of carboxymethyl chitosan: Take 5g of chitosan and add it to 35mL of isopropanol, stir and swell for 2h, then dissolve 25g of sodium hydroxide in 25mL of water and pour it into the above-mentioned swollen solution to obtain alkali Chitosan-isopropanol system. After stirring evenly, place it at -18°C for 12 hours. Weigh 17.61g of chloroacetic acid, heat and dissolve it in 10mL of isopropanol, add it into the above-mentioned alkaline chitosan-isopropanol system in five batches under ice bath, and then react at 60°C for 5h, and then react backward Pour 100mL of water into the system to dissolve the solids in the reaction system, adjust the pH of the system to 8 with 10% hydrochloric acid, then pour the resulting solution into 800mL of absolute ethanol to precipitate, filter with suction, wash wit...
Embodiment 2
[0031] The difference from Example 1 is:
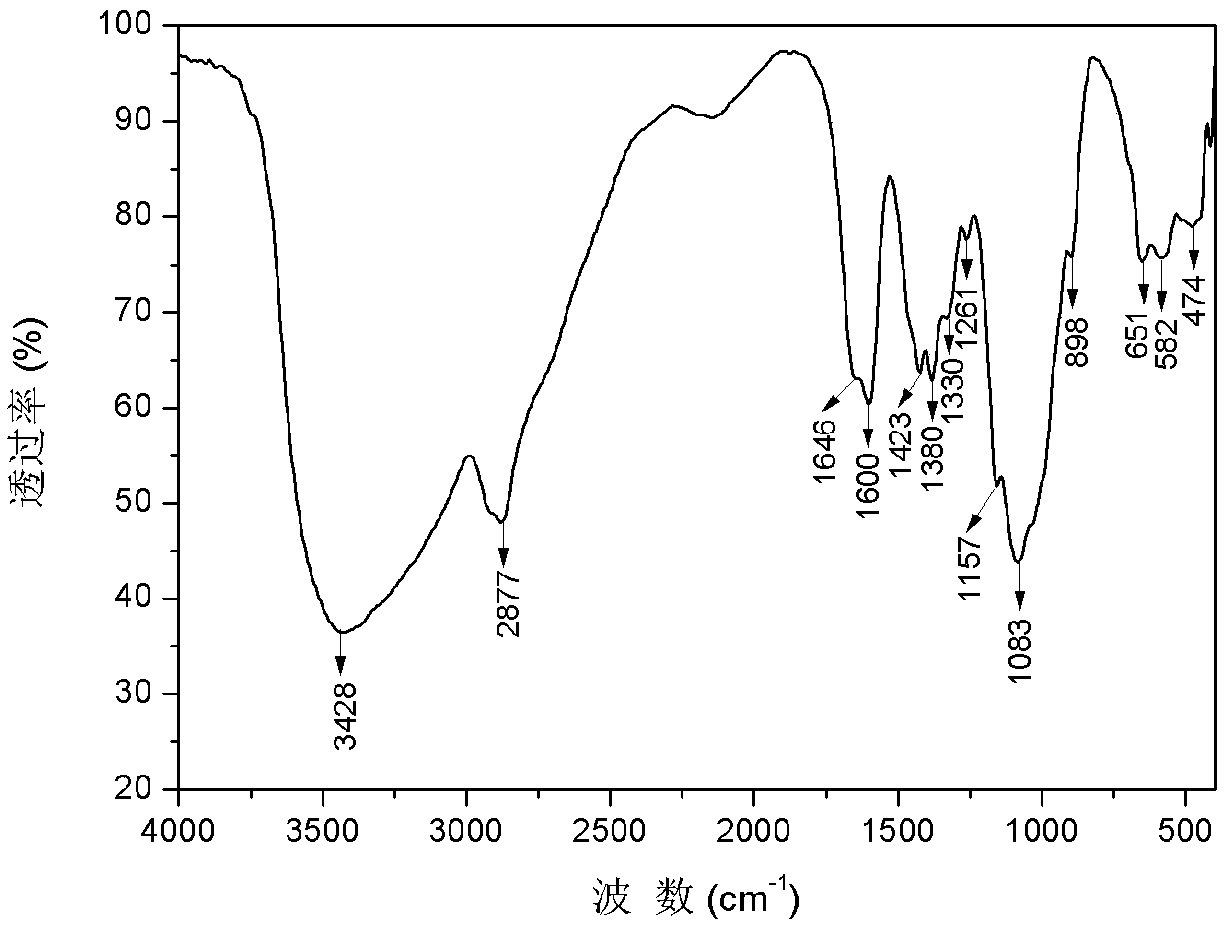
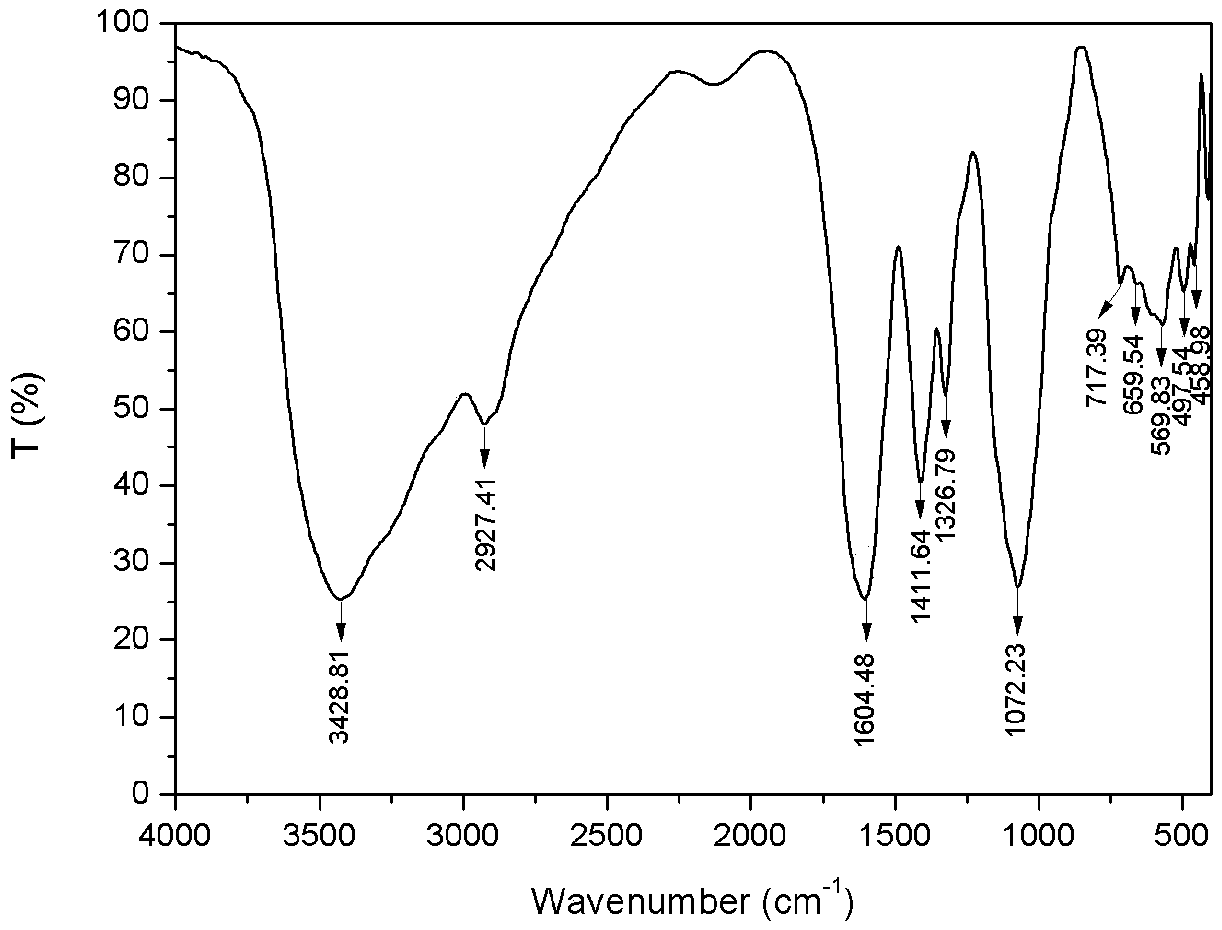
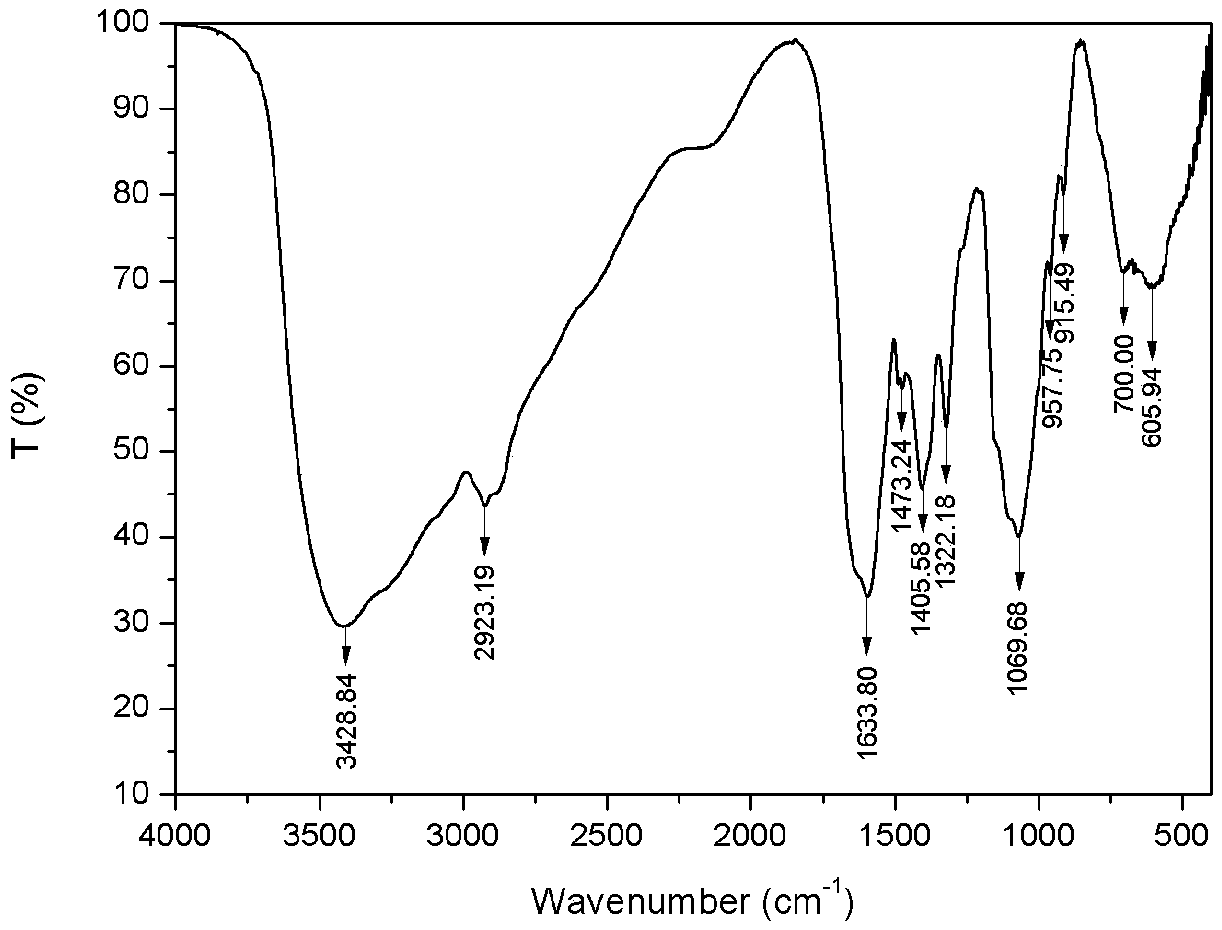
[0032] 1) Preparation of carboxymethyl chitosan: Weigh 5g of chitosan and add it to 40mL of isopropanol, stir and swell for 2h, then dissolve 30g of sodium hydroxide in 30mL of water and pour it into the above-mentioned swollen solution to obtain alkali Chitosan-isopropanol system. After stirring evenly, place it at -20°C for 10 hours. Weigh 14.67g of chloroacetic acid, heat and dissolve it in 10mL of isopropanol, add it into the above-mentioned alkaline chitosan-isopropanol system in five batches under ice bath, and then react at 50°C for 10h, and then react backward Pour 100mL of water into the system to dissolve the solids in the reaction system, adjust the pH of the system to 8 with 10% hydrochloric acid, pour the resulting solution into 800mL of absolute ethanol to precipitate, filter with suction, wash with absolute ethanol, and dry in vacuum at -50°C for 24h , to obtain carboxymethyl chitosan (see figure 2 ) 9.62g, spare. ...
Embodiment 3
[0035] The difference from Example 1 is:
[0036] 1) Preparation of carboxymethyl chitosan: Take 5g of chitosan and add it to 35mL of isopropanol, stir and swell for 2h, then dissolve 25g of sodium hydroxide in 25mL of water and pour it into the above-mentioned swollen solution to obtain alkali Chitosan-isopropanol system. After stirring evenly, place it at -20°C for 10 hours. Weigh 17.61g of chloroacetic acid, heat and dissolve it in 10mL of isopropanol, add it into the above-mentioned alkaline chitosan-isopropanol system in five batches under ice bath, and then react at 50°C for 10h, and then react backward Pour 100mL of water into the system to dissolve the solids in the reaction system, adjust the pH of the system to 8 with 10% hydrochloric acid, pour the resulting solution into 800mL of absolute ethanol to precipitate, filter with suction, wash with absolute ethanol, and dry in vacuum at -50°C for 24h , to obtain carboxymethyl chitosan (see figure 2 ) 10.51g, spare. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com