MiRNA marker of immune thrombocytopenia, kit and application
A technology for thrombocytopenia and immunity, applied in the field of medical testing, to achieve high sensitivity, improve stability, and avoid data deviation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 The method for detecting miRNA markers of immune thrombocytopenia
[0040] 1. Collection of clinical samples
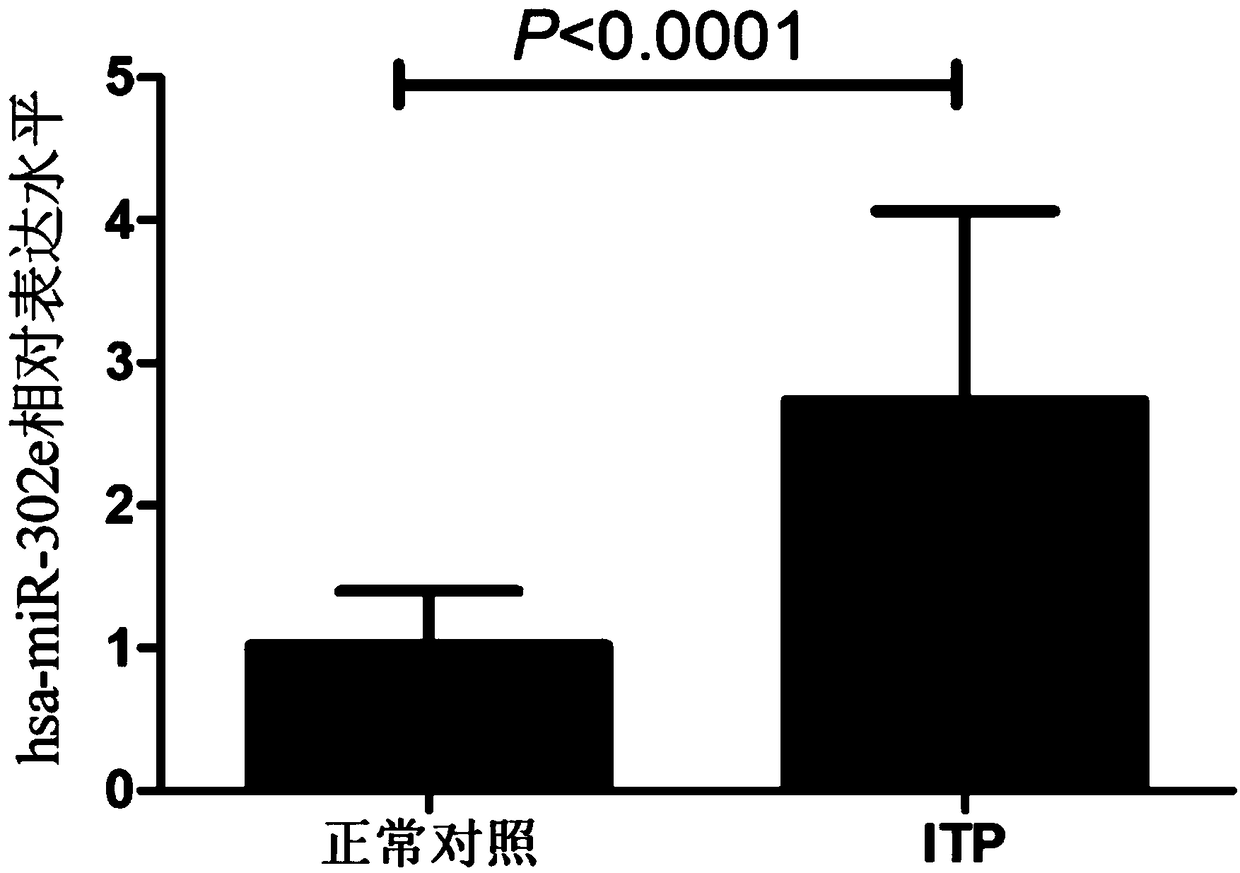
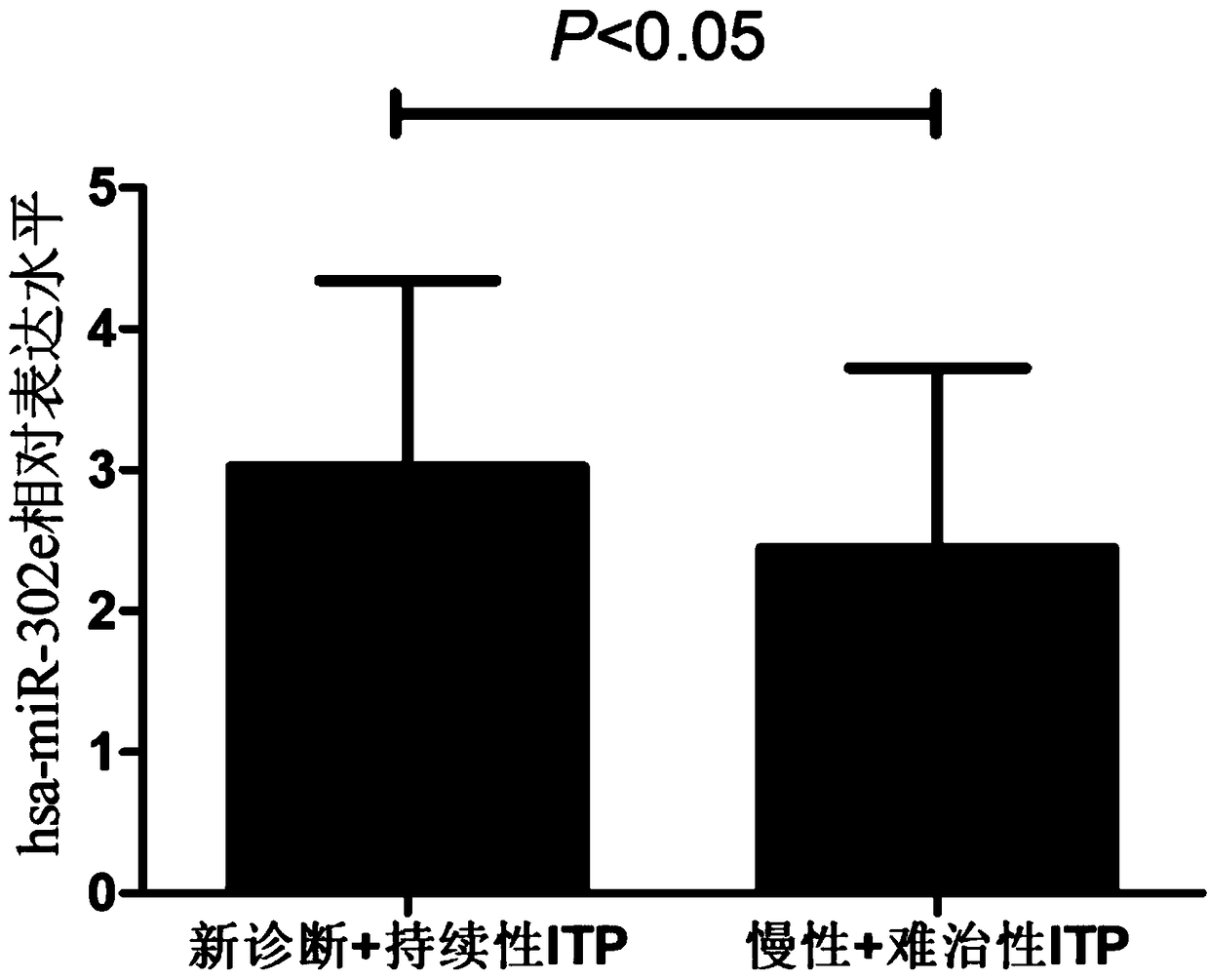
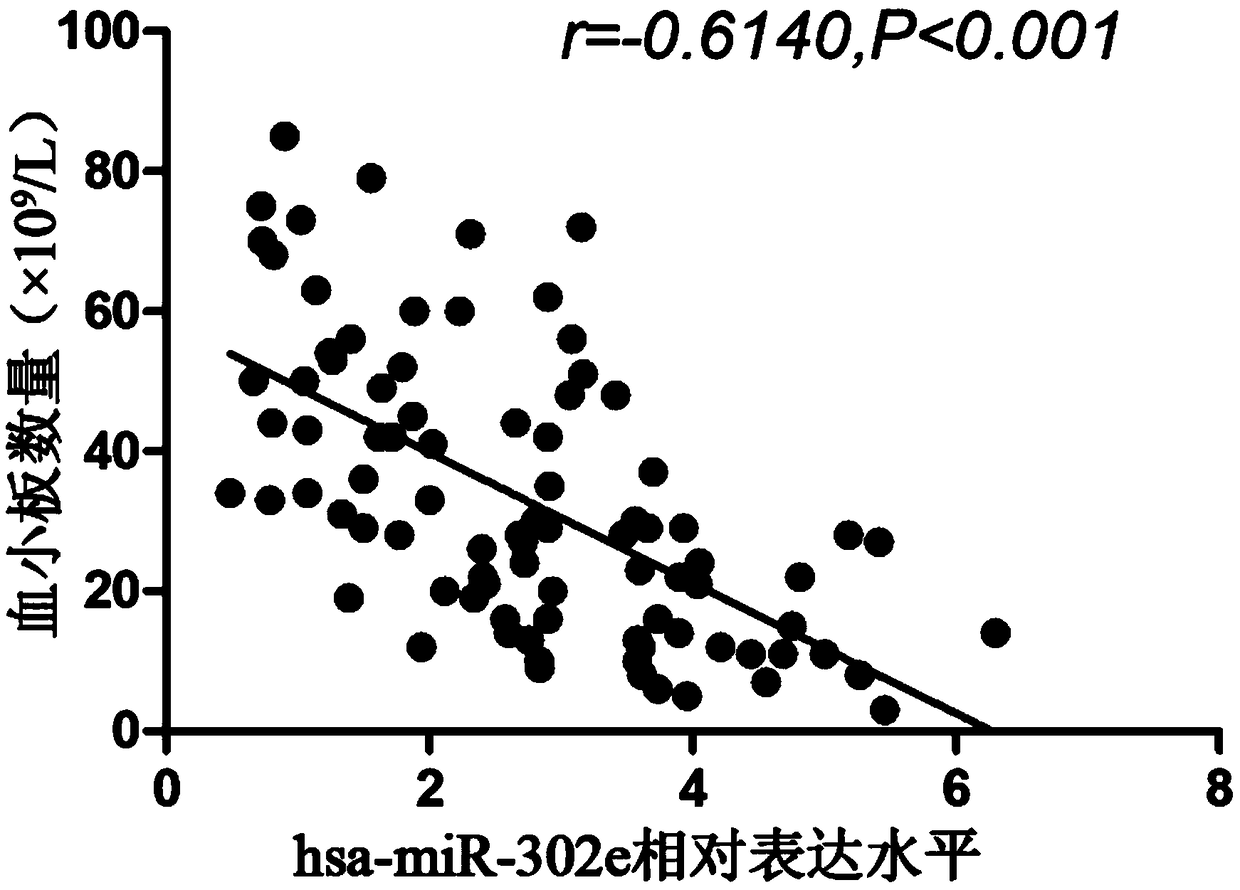
[0041]The inventor has collected a large number of patients with immune thrombocytopenia from the 100th Hospital of the People's Liberation Army and the Affiliated Hospital of Soochow University since 2016. The diagnostic criteria all meet the "Chinese Expert Consensus on the Diagnosis and Treatment of Primary Immune Thrombocytopenia in Adults (Revised Edition)"; At the same time, the healthy positive control group in the physical examination center was selected, and peripheral blood was drawn. This study was approved by the Hospital Ethics Committee, and all subjects signed informed consent. The process of collection and follow-up experiments complied with the requirements of medical ethics and strictly followed the principle of confidentiality of case data. The sampling, packaging and storage conditions of the research samples were the same. By a...
Embodiment 2
[0105] This implementation provides an auxiliary diagnostic kit for immune thrombocytopenia, which is used to detect the expression level of hsa-miR-302e in peripheral blood. The kit contains miRNA markers of immune thrombocytopenia; the base sequence of the miRNA markers is shown in SEQ ID NO:1. The kit also includes primers for miRNA markers, the base sequences of which are shown in SEQ ID NO: 2 and SEQ ID NO: 3. The kit also includes commonly used reagents for total RNA extraction, cDNA reverse transcription primers, commonly used reagents for reverse transcription, polymerase and reagents commonly used for fluorescent quantitative Q-PCR reactions and fluorescent detection reagents (see Example 1); the cDNA The reverse transcription primer sequence is shown in SEQ ID NO:4. The kit also includes a standard and / or a reference; the standard and / or a reference is endogenous miRNA-U6RNA.
[0106] Using the kit can achieve the same detection effect as the embodiment, and the op...
Embodiment 3
[0108] ROC curve was used to analyze the diagnostic value of hsa-miR-302e level for ITP. Among the 127 test subjects, with ITP patients as the target subjects, the ROC curve was drawn using Graphpad software, and various related parameters were analyzed. Such as Figure 4 As shown, the area under the curve reaches 0.8913, indicating that it has a very good diagnostic value. Using the principle of the largest Youden's index, the optimal cut-off value of hsa-miR-302e was judged to be 1.552, and the corresponding sensitivity and specificity were 77.54 and 95%, respectively, as shown in Table 2.
[0109] Table 2:
[0110]
[0111]
[0112] Depend on Figure 4 It can be seen from Table 2 that the area under the curve is 0.0.891±0.027 (P<0.001), suggesting that it has a high diagnostic value. The optimal cut-off value of hsa-miR-302e concentration was 1.552, and the corresponding sensitivity and specificity were 77.53% and 95%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com