Chemical synthesis method of 3-isoxazolyl benzyl acetate
A technology of isoxazolyl and benzyl acetate, which is applied in the field of chemical synthesis of 3-isoxazolyl benzyl acetate, can solve problems such as difficulty in obtaining, unfavorable production, and high production cost requirements, and achieve the effect of guaranteed yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
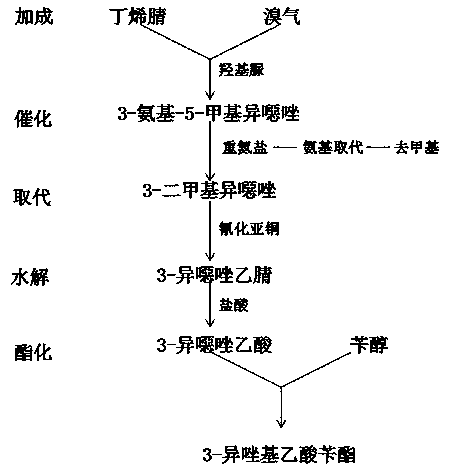
[0017] refer to figure 1 , a chemical synthesis method of 3-isoxazolyl benzyl acetate, comprising the following steps:
[0018] S1: Addition reaction of butenenitrile and bromine gas to generate 3-amino-5-methylisoxazole under the action of derivatives of hydroxylamine;
[0019] S2: Then 3-amino-5-methylisoxazole and 3-amino-5-methylisoxazole are catalyzed to produce diazonium salt at low temperature, and the diazonium salt is subjected to a substitution reaction under hydrochloric acid environment to replace the amino group to produce 3, 5-Dimethylisoxazole, demethylated to produce 3-chloromethylisoxazole;
[0020] S3: Add 3-chloromethylisoxazole and cuprous cyanide to an organic solvent, the organic solvent is dimethyl sulfoxide, and react to prepare 3-isoxazole acetonitrile;
[0021] S4: Add 3-isoxazole acetonitrile to hydrochloric acid, and hydrolyze to obtain 3-isoxazole acetic acid;
[0022] S5: Add 3-isoxazoleacetic acid and benzyl alcohol into dichloromethane or tri...
Embodiment 1
[0024] Embodiment 1: 3-chloromethyl isoxazole synthesis:
[0025] Using chloroacetyl chloride and acetylene gas as raw materials, anhydrous aluminum trichloride as a catalyst, and methylene chloride as a solvent to obtain 1,4-dichloro-3-butene-2-one; then 1,4-dichloro - 3-buten-2-one and hydroxylamine hydrochloride are refluxed and closed in methanol solvent to obtain 3-chloromethylisoxazole, and the specific steps for synthesizing 1,4-dichloro-3-buten-2-one are 110g , chloroacetyl chloride with a molar mass of 1.06 was slowly added dropwise to 150 g of anhydrous AlCl with a molar mass of 1.17 3 and 300ml of CH 2 Cl 2 In the mixed solution, the mixture was cooled to -5°C, acetylene gas was introduced for 2 hours, the reactant was slowly poured into ice water, the organic layer was washed with cold water, dried over anhydrous sodium sulfate, and collected under reduced pressure at 78-83°C / 16mmHg Distillate oil 64g of 2,4-dichloro-2-buten-2-one, the specific steps of synthesi...
Embodiment 2
[0032] Embodiment 2: Synthesis of 3-chloromethylisoxazole
[0033] Use acetylacetonate and hydroxylamine to generate 5-methylisoxazole-3-carboxylate, then perform nucleophilic addition with ammonia to generate 5-methylisoxazole-3-carboxamide, and then dealcoholize The product 3-amino-5-methylisoxazole is produced, and then 3-amino-5-methylisoxazole is catalyzed to make diazonium salt at low temperature, and the diazonium salt is prepared under hydrochloric acid environment A substitution reaction occurs, and the amino group is substituted to produce 3,5-dimethylisoxazole, and demethylated to produce 3-chloromethylisoxazole.
[0034] Add dimethyl sulfoxide and 3-chloromethylisoxazole into the three-necked flask, add cuprous cyanide, heat to 30°C, after the reaction is complete, add water, extract the combined organic phase with dichloromethane, and wash with saturated bicarbonate Wash with sodium, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com