Glycyrrhetinic acid derivative and its preparation method and use
A technology of glycyrrhetic acid and derivatives, which can be used in drug combinations, pharmaceutical formulations, steroids, etc., can solve problems such as poor water solubility and poor bioavailability, and achieve the effects of improving antitumor activity and inhibiting adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1. PEG 350 Synthesis of monomethyl ether glycyrrhetinic acid (GTA-mPEG 350 )
[0049]
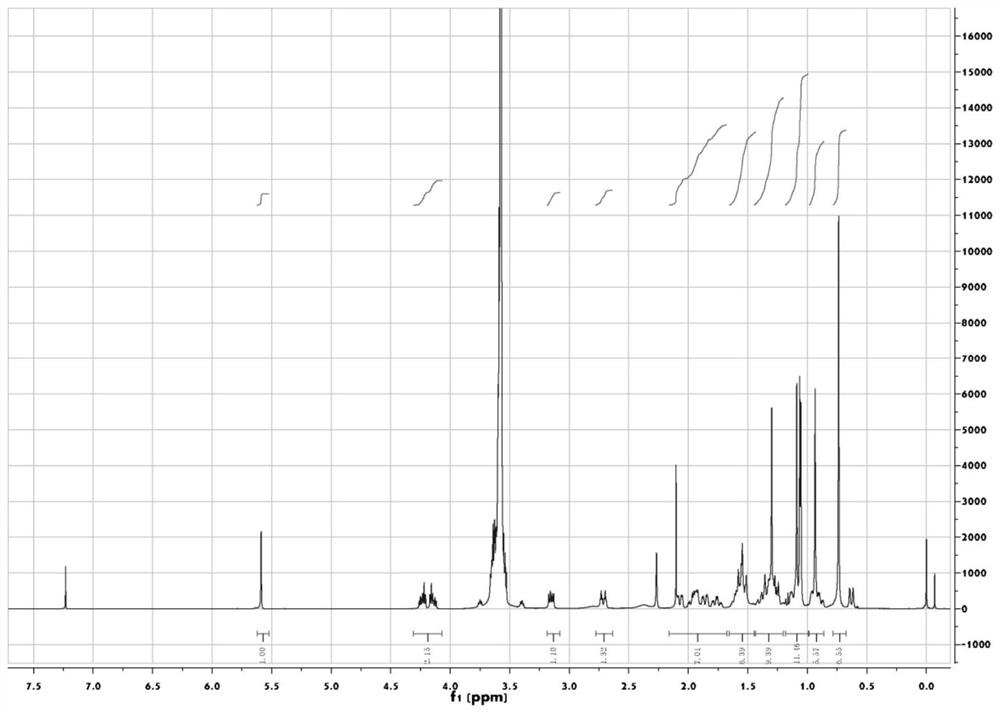
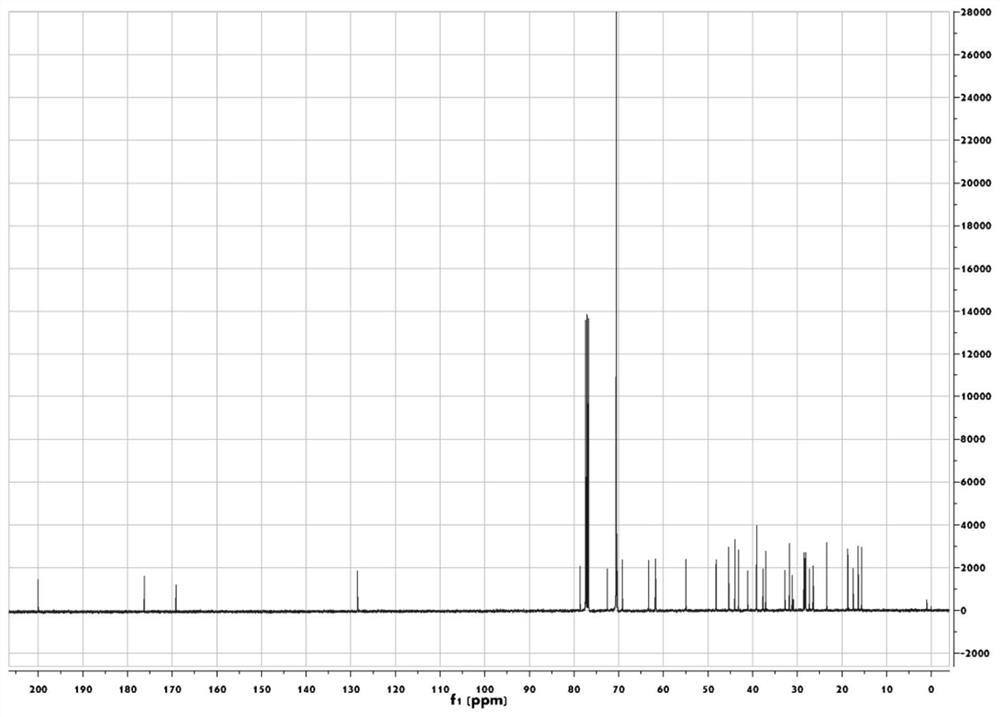
[0050] Glycyrrhetinic acid (330mg, 0.7mmol) and PEG 350 Monomethyl ether (compound represented by formula (4) where n is 8 and R is methyl, 280 mg, 0.8 mmol) was dissolved in 40 mL of dichloromethane. EDC-HCl (153 mg, 0.8 mmol) and DMAP (9.4 mg, 0.08 mmol) were added sequentially, and stirred at room temperature for 14 hours. After the reaction was complete, aqueous hydrochloric acid (0.1N, 50 mL) was added, stirred at room temperature for 10 minutes, and then extracted with dichloromethane (20 mL) for a total of three extractions. The extracts were combined and washed with saturated brine (30 mL). Anhydrous Na was added to the organic phase 2 SO 4 After drying, the organic solvent is distilled off under reduced pressure after filtration, and the thick product is separated through silica gel chromatography (eluent is V 石油醚 :V 乙酸乙酯 :V 乙酸 =20:10:1) to get the compou...
Embodiment 2
[0051] Example 2. PEG 400 Glycyrrhetinic acid (GA-I-PEG 400 )
[0052]
[0053] Synthesize according to the synthesis method of Example 1. use PEG 400 (n is 9 and R is the compound shown in the formula (4) of H) replaces the PEG of embodiment 1 350 monomethyl ether. Compound GTA-PEG 400 The yield is 75%.
Embodiment 3
[0054] Example 3.PEG 1000 Glycyrrhetinic acid (GTA-PEG 1000 )
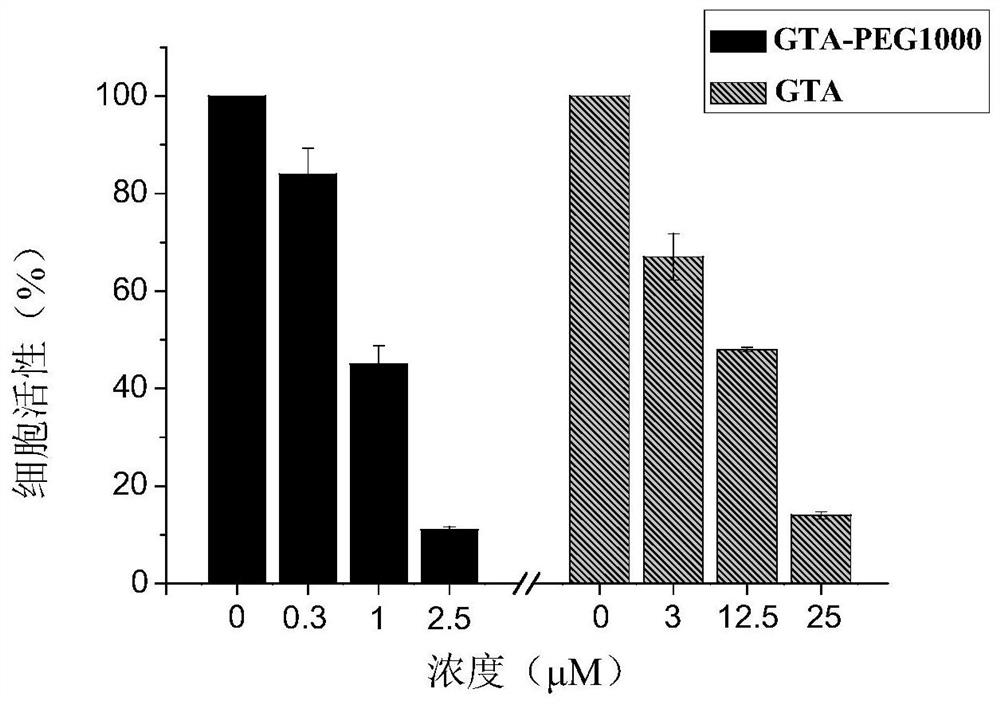
[0055] Synthesize according to the synthesis method of Example 1. use PEG 1000 (n is 23 and R is the compound shown in the formula (4) of H) replaces the PEG of embodiment 1 350 monomethyl ether. Compound GTA-PEG 1000 Yield 70%.
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com