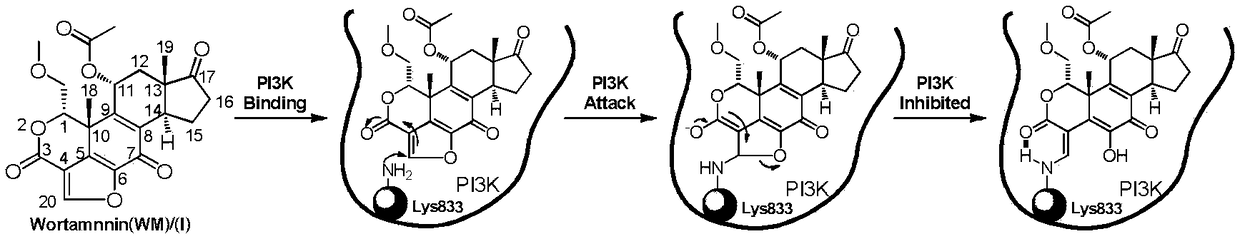
Wortmannin prodrug as well as preparation and application thereof
A wortmannin and prodrug technology, applied in the field of wortmannin prodrug and preparation, can solve the problems of restricting the development and utilization of wortmannin, enhancing toxicity, reducing stability, etc., so as to improve anti-tumor targeting and reduce dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 compound (II-1)
[0035] In a 25mL round-bottomed flask, compound I (10mg, 23μmol) and triethylamine (16μL, 100μmol) were dissolved in 10mL of dichloromethane, then compound III (9mg, 28μmol) was added, stirred at room temperature for 10 hours, and vacuum Drain the solvent, use high-efficiency thickening to prepare thin-layer plates for separation, the developing agent is dichloromethane:methanol:triethylamine with a volume ratio of 20:1:0.1, collect samples with an Rf value of 0.6, and obtain the target compound (II-1 ) (7mg, 43%), see Figure 4 .
[0036] 1 H NMR(500MHz,DMSO)δ8.69(s,1H),8.02(s,1H),6.42(s,1H),6.37(s,1H),5.95(dd,J=7.1,4.6Hz,1H) ,4.31(t,J=6.5Hz,2H),4.14(s,1H),3.64-3.73(m,3H),3.04-3.22(m,8H),2.90-3.04(m,3H),2.75-2.84 (ddd,J=19.5,11.7,5.5Hz,3H),2.53-2.63(m,3H),2.20-2.38(m,5H),1.99-2.09(m,4H),1.76(d,J=13.9Hz ,1H),1.42-1.68(m,8H),1.27-1.40(s,2H),0.78(s,3H).HRMS(ESI):m / z[M+Na] + calcd 763.2989, found: 763.3017.
[0037] ...
Embodiment 2
[0038] Preparation of Example 2 Compound (II-2)
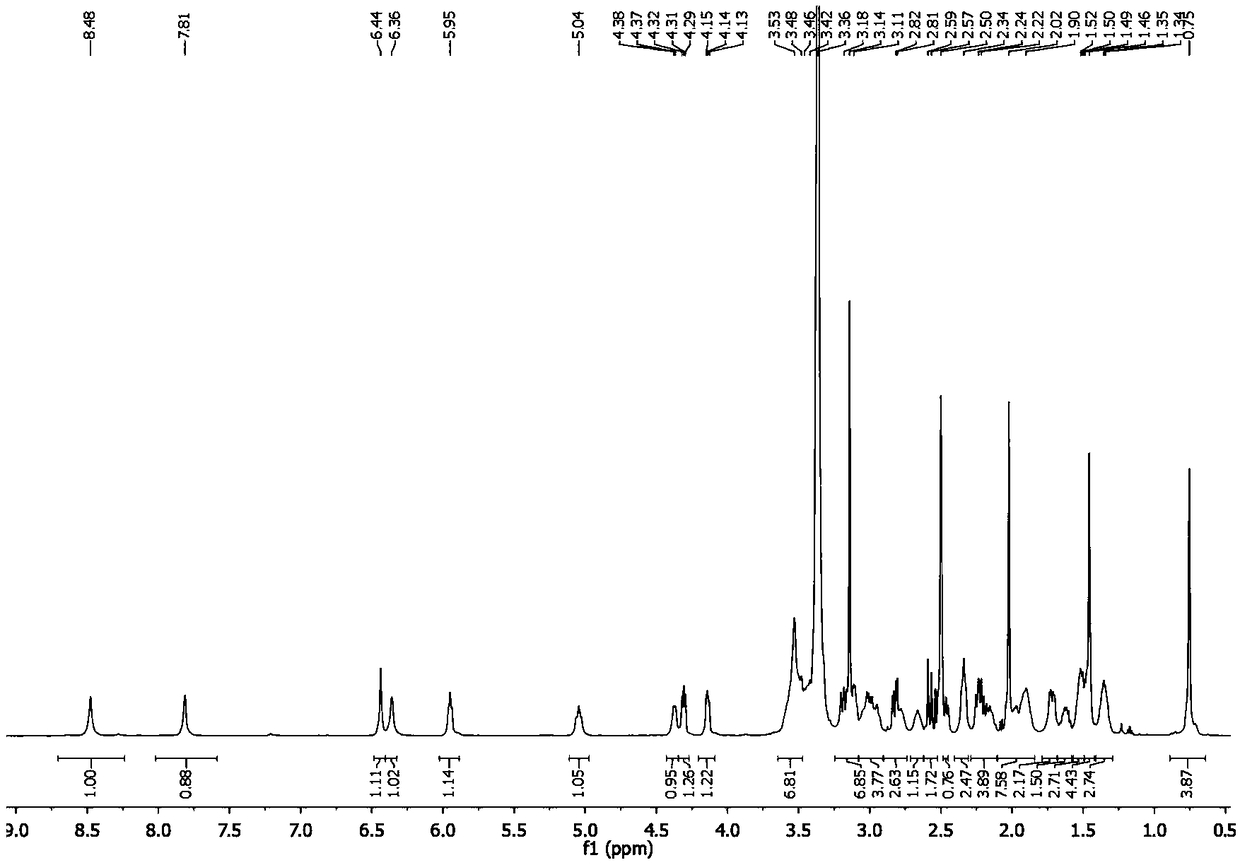
[0039] In a 25mL round-bottomed flask, compound I (10mg, 23μmol) and triethylamine (16μL, 100μmol) were dissolved in 10mL of dichloromethane, compound IV (11mg, 28μmol) was added, stirred at room temperature for 10 hours, and vacuum Drain the solvent, use high-efficiency thickening to prepare thin-layer plates for separation, the developing agent is dichloromethane:methanol:triethylamine with a volume ratio of 12:1:0.1, collect samples with an Rf value of 0.5, and obtain the target product (II-2 ) (10mg, 52%), see Figure 5 .
[0040] 1H NMR(500MHz,DMSO)δ8.48(s,1H),7.81(s,1H),6.44(s,1H),6.36(s,1H),5.95(t,J=5.66Hz,1H),5.04 (t,J=8.92Hz,1H),4.37(d,J=5.5Hz,1H),4.27-4.34(m,1H),4.08-4.19(m,1H),3.44-3.66(m,7H), 3.04-3.20(m,7H),2.91-3.09(m,4H),2.74-2.87(m,3H),2.66(s,1H),2.52-2.62(m,2H),2.42-2.46(m,1H ),2.34(s,2H),2.12-2.27(m,4H),1.86-2.08(m,7H),1.70-1.78(m,2H),1.57-1.65(m,2H),1.42-1.54(m ,7H),1.29-1.41(m,3H),0.75(s,4H).HRMS(ESI):...
Embodiment 3
[0042] Preparation of Example 3 Compound (II-3)
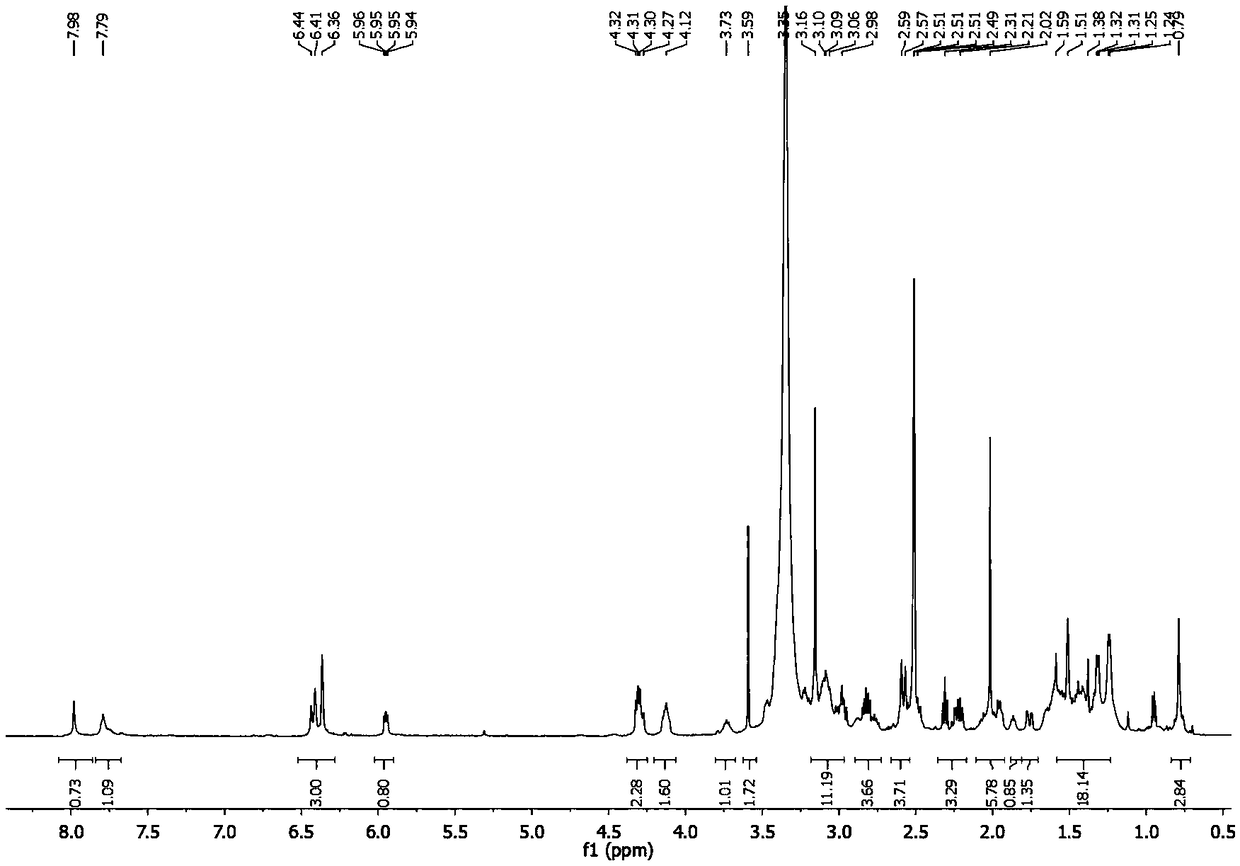
[0043] In a 25 mL round bottom flask, compound I (10 mg, 23 μmol) and triethylamine (16 μL, 100 μmol) were dissolved in 10 mL of dichloromethane, compound V (9 mg, 28 μmol) was added, stirred at room temperature for 10 hours, and vacuum Drain the solvent, and use high-efficiency thickening to prepare thin-layer plates for separation. The developing agent is dichloromethane:methanol:triethylamine with a volume ratio of 10:1:0.1, and samples with an Rf value of 0.23 are collected to obtain the target product (II-3 ) (9mg, 46%), see Figure 6 .
[0044] 1 H NMR (500MHz, DMSO) δ7.98(s, 1H), 7.79(s, 1H), 6.32-6.47(m, 3H), 5.95(dd, J=7.3, 4.5Hz, 1H), 4.30(dd, J=15.4,9.6Hz,2H),4.12(s,2H),3.73(s,1H),3.59(s,2H),2.94-3.19(m,11H),2.71-2.89(m,3H),2.53 -2.63(m,5H),1.93-2.18(m,6H),1.87(s,1H),1.76(dd,J=14.3,4.0Hz,1H),1.18-1.58(m,18H),0.78(s ,3H).HRMS(ESI):m / z[M+Na] + calcd 779.3302, found: 779.3310.
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com