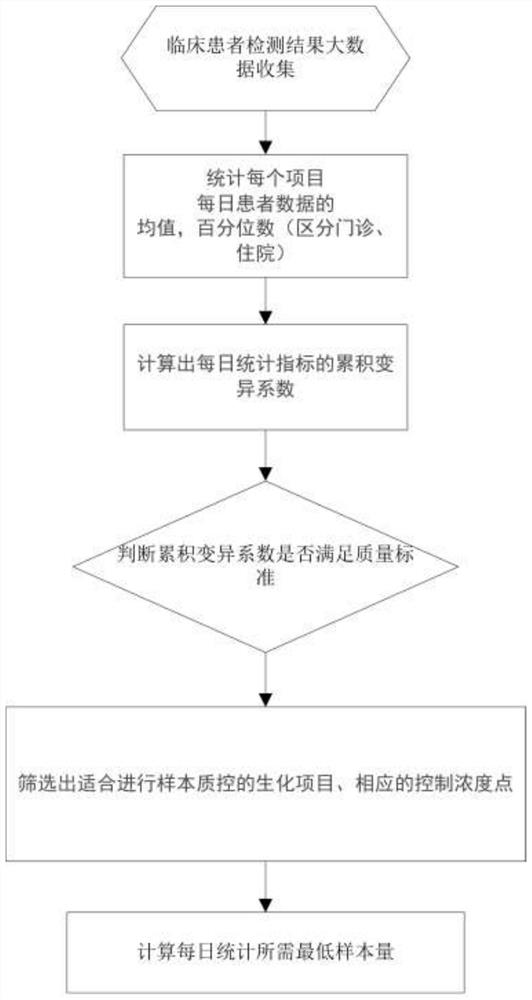
Biochemical detection quality control method
A quality control method and biochemical detection technology, applied in the field of medical examination, can solve the problems of inability to detect process monitoring, and achieve the effect of low cost, high quality and good supplementary effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] For outpatients: AFU, APOA, APOB, CA, CL, HDL, K, MG, NA, NEFA, TP, and URIC are used As control points, the statistical minimum sample sizes are 22, 17, 20, 9, 11, 19, 39, 10, 8, 21, 14 and 24, and the control point concentrations are 29.18, 1.41, 0.84, 2.31, 102.78, 1.29 , 4.21, 0.86, 140.82, 507.66, 72.10 and 333.96, the standard deviations of control point concentrations were 1.46, 0.06, 0.04, 0.03, 0.56, 0.06, 0.04, 0.02, 0.61, 33.42, 1.09 and 13.79; The digit P25 is used as the control point, the concentration of the control point is 69.83 and 9.90, and the standard deviation of the concentration of the control point is 3.37 and 0.52 respectively; AST, GLU, GPDA and PHOS use the percentile P50 as the control point, and the concentration of the control point is 23.15 , 5.31, 86.83, and 1.13, and the standard deviations of the control point concentrations were 1.21, 0.15, 6.13, and 0.03, respectively; 3.04, and the standard deviations of the control point concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com