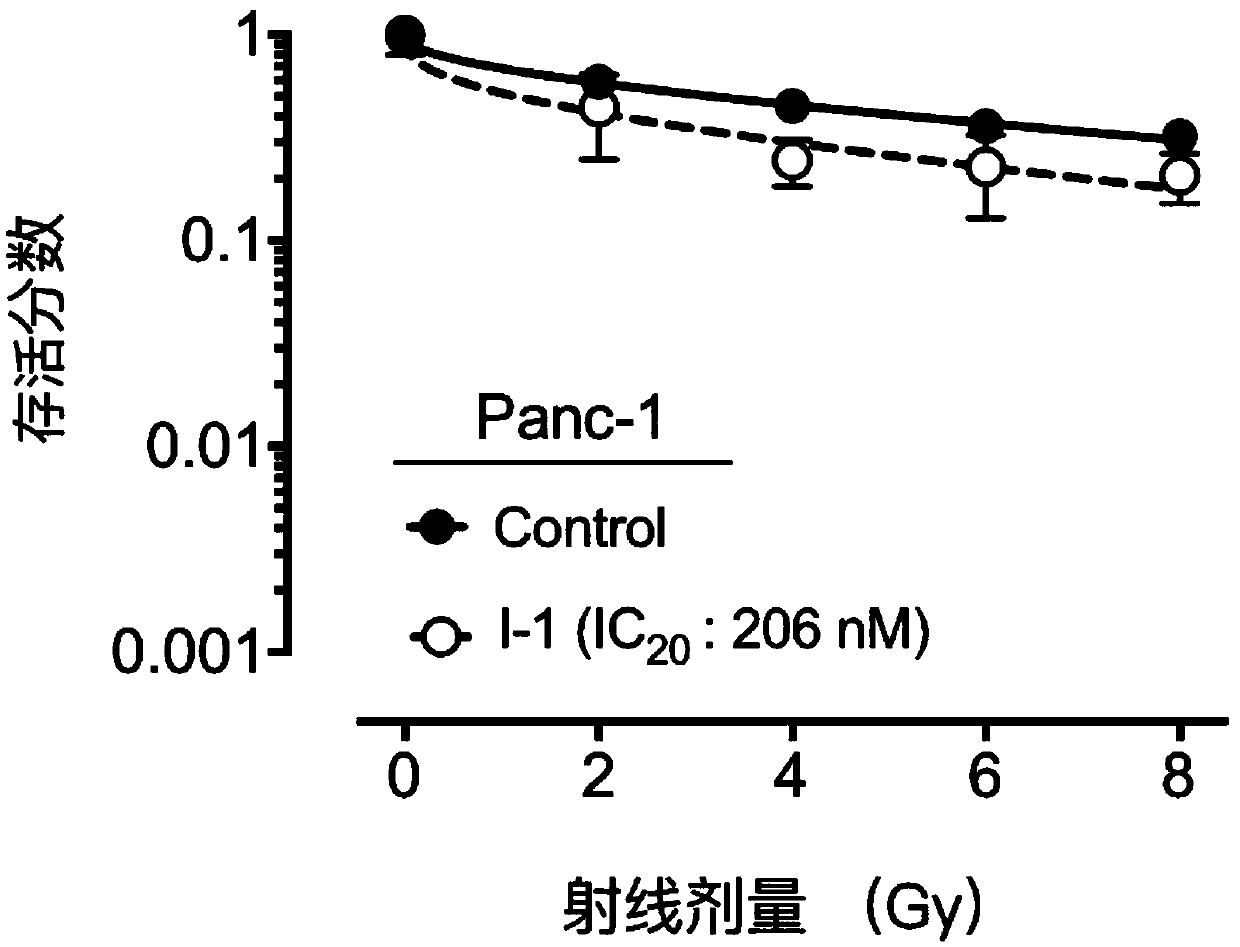
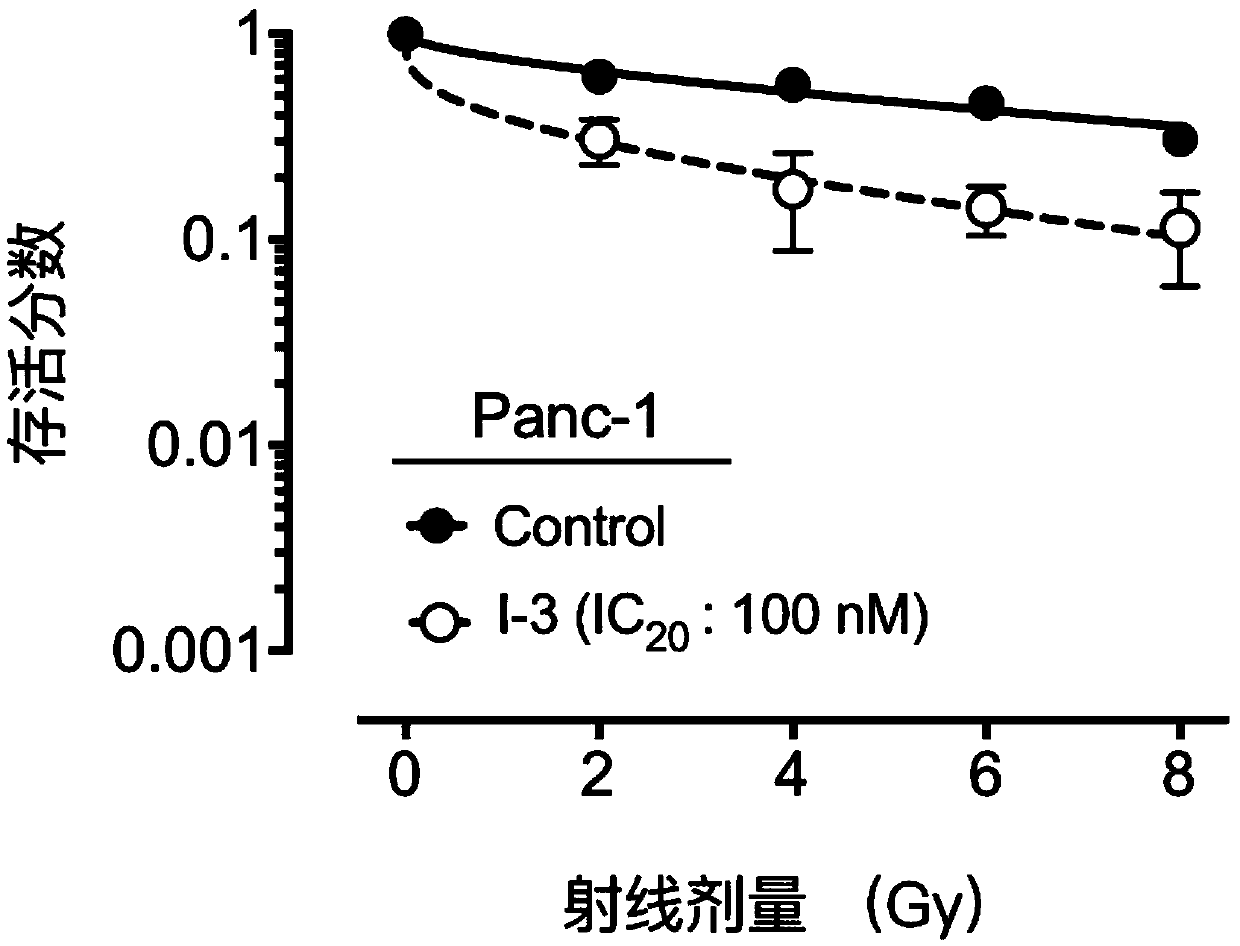
Compound containing heptatomic lactam ring and application of compound
A technology of lactam ring and compound, applied in the field of medicine, can solve the problems of high local recurrence and radiation resistance, and achieve the effect of improving the sensitivity of radiotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of 3-chloro-1-phenylallyl-6,7-dihydro-1H-azepine-2(5H)-one, the synthetic route is as follows:
[0049]
[0050] Weigh 62 mg of cinnamic acid 1 and dissolve in anhydrous dichloromethane (2 mL), add oxalyl chloride (0.18 mL) and a catalytic amount of DMF. After stirring at room temperature for 2 hours, the solvent was evaporated under reduced pressure to obtain compound 2, which was directly used in the next step.
[0051] The compound 3-chloro-6,7-dihydro-1H-azepine-2(5H)-one (3,68mg) was dissolved in anhydrous tetrahydrofuran (5mL), cooled to -78°C and slowly added n-butyl Lithium tetrahydrofuran solution (2.5M, 0.2mL), continued the reaction for 15 minutes, and then added dropwise the anhydrous tetrahydrofuran solution of compound 2 obtained in one step. Slowly rise to room temperature and react for 30 minutes, quenched, extracted with dichloromethane, dried, and evaporated under reduced pressure to obtain a crude product, which was separated by chro...
Embodiment 2
[0053] (E)-N-(4-(3-(6-Chloro-7-oxo-2,3,4,7-tetrahydro-1H-azaheptacycline-1-yl)-3-oxopropene- 1-base) the preparation of phenyl) acrylamide, synthetic route is as follows:
[0054]
[0055] Weigh 62 mg of compound 4 and dissolve in anhydrous dichloromethane (2 mL), add oxalyl chloride (0.18 mL) and a catalytic amount of DMF. After stirring at room temperature for 2 hours, the solvent was evaporated under reduced pressure to obtain compound 5, which was directly used in the next step.
[0056] The compound 3-chloro-6,7-dihydro-1H-azepine-2(5H)-one (3,68mg) was dissolved in anhydrous tetrahydrofuran (5mL), cooled to -78°C and slowly added n-butyl Lithium tetrahydrofuran solution (2.5M, 0.2mL), continued to react for 15 minutes, and then added dropwise the anhydrous tetrahydrofuran solution of compound 5 obtained in one step. Slowly rise to room temperature and react for 30 minutes, quenched, extracted with dichloromethane, dried, evaporated the solvent under reduced pressure...
Embodiment 3
[0060] (E)-2-Chloro-N-(4-(3-(6-Chloro-7-oxo-2,3,4,7-tetrahydro-1H-azaheptane-1-yl)-3- Preparation of oxypropen-1-yl) phenyl) acetamide:
[0061]
[0062] Dissolve Intermediate 7 (57 mg) in dichloromethane (10 mL), add 18 μL of triethylamine, react at 0°C for 10 minutes, slowly add 35 μL of chloroacetyl chloride, react at 0°C for 4 hours, extract with dichloromethane, wash with water , separated by chromatographic column to obtain compound I-3, light yellow solid, yield, 68%. 1 H NMR (300MHz, CDCl 3 )δ: 1.98-2.04 (m, 2H, J=6.3Hz, CH 2 ), 2.34-2.41 (q, 2H, J=7.0Hz, CH 2 ),3.97-4.02(t,2H,J=6.4Hz,NCH 2 ), 5.78-5.82 (d, 1H, J = 10.5Hz, = CH), 6.21-6.30 (dd, 1H, J 1 =10.0Hz,J 2 =10.1Hz, =CH), 6.43-6.49(d,1H,J=17.0Hz,=CH),6.76-6.81(t,1H,J=7.8Hz,=CH),7.39-7.44(d,2H, J=15.5Hz, Ar-H), 7.53-7.56(d, 2H, J=8.6Hz, Ar-H), 7.60-7.64(d, 2H, J=8.7Hz, Ar-H), 7.74-7.79( d,1H,J=15.5Hz,=CH); 13 C NMR (75MHz, CDCl 3 )δ: 23.82, 26.04, 41.45, 118.99, 119.97, 128.61, 129.60, 130.99, 131.05...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com