A kind of hemostatic sponge and preparation method thereof
A technology of hemostatic sponge and sponge, which is applied in the field of medical devices, can solve the problems of no degradation, low compressive support strength, etc., and achieve obvious effects and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
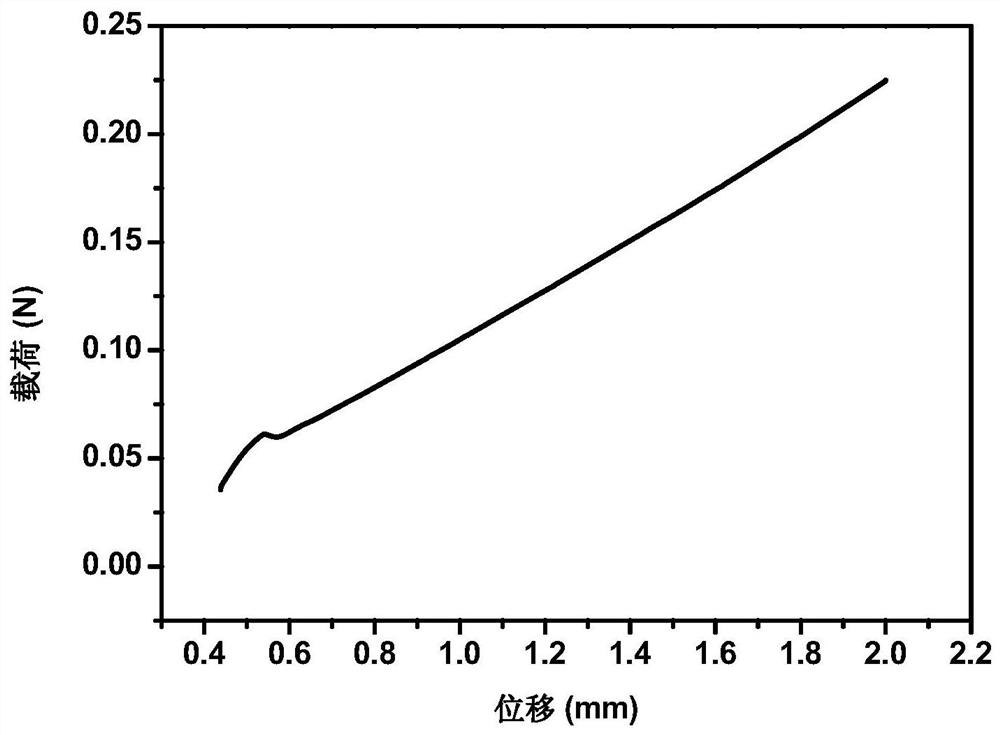
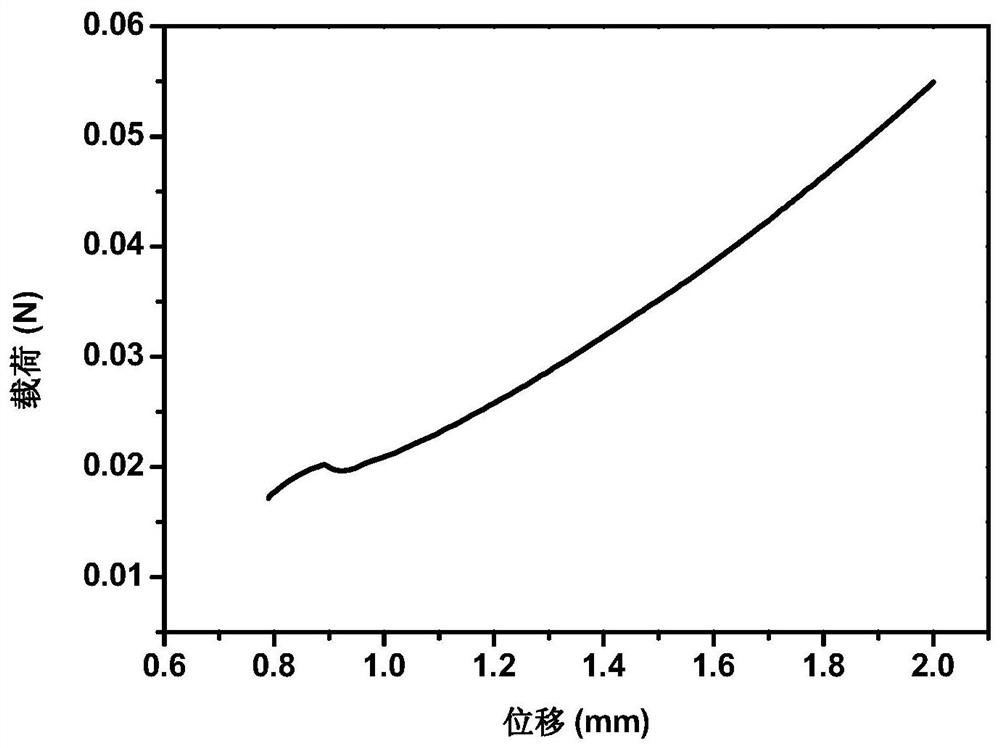
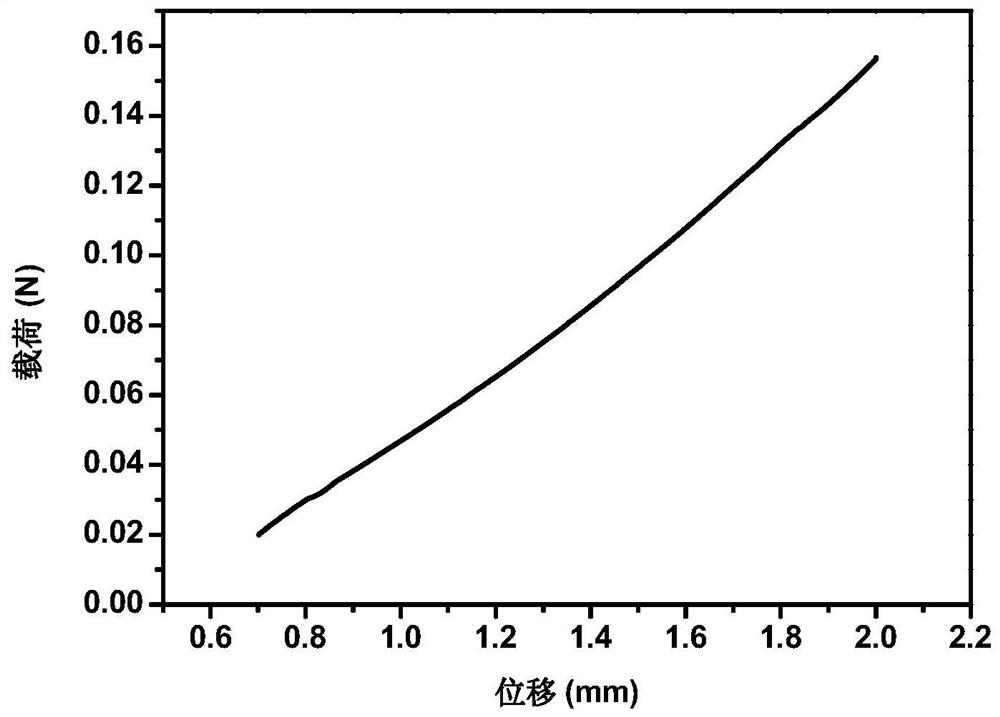
Image
Examples
Embodiment 1
[0055] Accurately weigh 2 g of SA, place it in 100 mL of distilled water, and stir until completely dissolved. Add 1g CS to the above SA solution, so that the concentration of CS is 1% (mass percentage), and continue to stir for 2h until the CS particles are uniformly mixed in the SA solution, and add 2mL of 10% acetic acid to the mixed solution (so that the final volume concentration of acetic acid is 0.5 %), stir evenly, inject the mixed solution into the mold, pre-freeze at -20°C for 3-4h, and vacuum-dry at 0-10°C for 24h to obtain a freeze-dried sponge. Place it in a coagulation bath and soak for 2 hours to obtain a sodium alginate-chitosan composite sponge, wherein the coagulation bath contains calcium chloride with a mass concentration of 1%, and a glycerol content of 2%. The sponge is dried in an oven to remove ethanol in the sponge to obtain a hemostatic sponge.
Embodiment 2
[0057] Accurately weigh 4 g of SA, place it in 100 mL of distilled water, and stir until completely dissolved. Add 1g CS to the above SA solution, so that the concentration of CS is 1% (mass percentage), and continue to stir for 2h until the CS particles are uniformly mixed in the SA solution, and add 2mL of 10% acetic acid to the mixed solution (so that the final volume concentration of acetic acid is 0.5 %), stir evenly, inject the mixed solution into the mold, pre-freeze at -20°C for 3-4h, and vacuum-dry at 0-10°C for 24h to obtain a freeze-dried sponge. Place it in a coagulation bath and soak for 2 hours to obtain a sodium alginate-chitosan composite sponge, wherein the coagulation bath contains calcium chloride with a mass concentration of 1%, and a glycerol content of 2%. The sponge is dried in an oven to remove ethanol in the sponge to obtain a hemostatic sponge.
Embodiment 3
[0059] Accurately weigh 2 g of SA, place it in 100 mL of distilled water, and stir until completely dissolved. Add 1g CS to the above SA solution, so that the concentration of CS is 1% (mass percentage), and continue to stir for 2h until the CS particles are uniformly mixed in the SA solution, and add 2mL of 10% acetic acid to the mixed solution (so that the final volume concentration of acetic acid is 0.5 %), stir evenly, inject the mixed solution into the mold, pre-freeze at -20°C for 3-4h, and vacuum-dry at 0-10°C for 24h to obtain a freeze-dried sponge. Place it in a coagulation bath and soak for 2 hours to obtain a sodium alginate-chitosan composite sponge, wherein the coagulation bath contains a calcium chloride mass concentration of 0.1%, a glycerol content of 2%, and a large amount of absolute ethanol after washing and crosslinking The sponge is dried in an oven to remove ethanol in the sponge to obtain a hemostatic sponge.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com