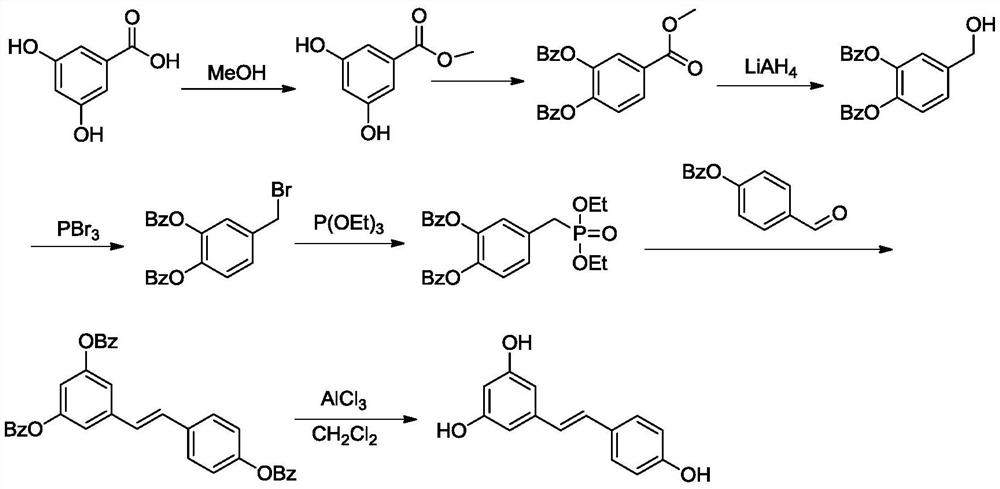
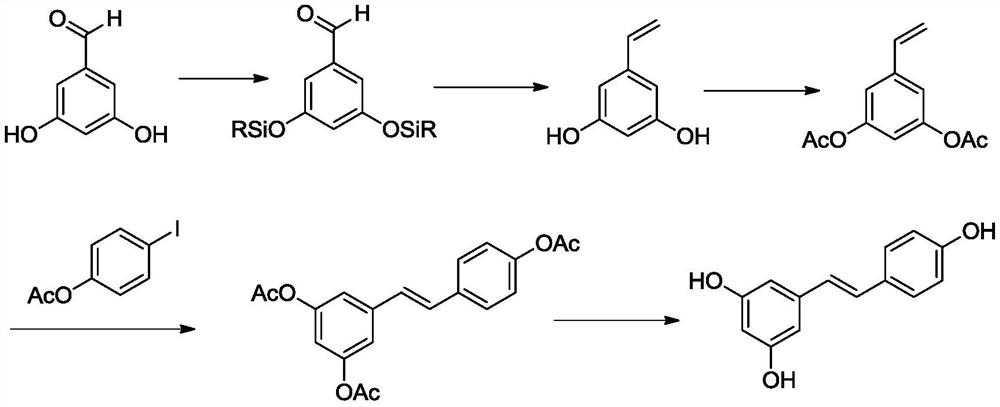
A kind of preparation method of resveratrol
A technology for resveratrol and compound, which is applied in the field of organic compound synthesis and can solve the problems of serious environmental pollution, expensive raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
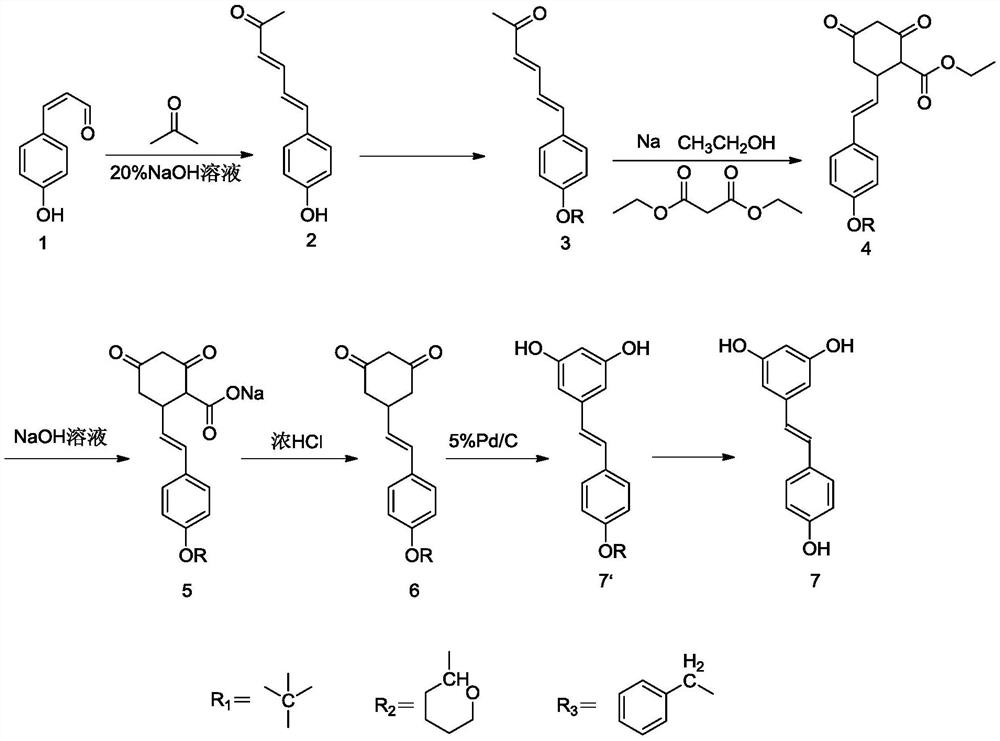
[0039] The synthetic route of resveratrol (1) is:
[0040]
[0041] Example 1
[0042] The synthetic method of resveratrol (7), the steps are as follows:
[0043] 1) Preparation of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one (compound 2)
[0044] Mix 1 g of p-hydroxycinnamaldehyde, 10 ml of acetone, and 2 ml of 20% sodium hydroxide solution, and stir at room temperature for 3 h. Add water to precipitate a solid, filter and wash with water to obtain the product 6-[4-hydroxyphenyl]-3,5-hexadien-2-one with a yield of over 95%.
[0045] The experimental data are: 1H NMR (400MHz, dmso) δ9.83(s, 1H), 7.42–7.29(m, 2H), 7.00(d, J=15.5Hz, 1H), 6.89(d, J=10.6Hz ,1H),6.77–6.72(m,2H),6.13(d,J=15.5Hz,1H),2.21(s,3H).
[0046] 2) Preparation of 6-[4-phenylbenzyloxy]-3,5-hexadien-2-one (compound 3)
[0047] Mix 1 g of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one, 12 ml of tetrahydrofuran, 0.54 g of potassium carbonate, and 0.72 g of benzyl bromide, stir at reflux at 70°C for 3 h, and quench with...
Embodiment 2
[0062] 1) Preparation of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one (compound 2)
[0063] Mix 5g of p-hydroxycinnamaldehyde, 40ml of acetone, and 9ml of 20% sodium hydroxide solution, and stir at room temperature for 3h. Add water to precipitate a solid, and wash with suction to obtain 6-[4-hydroxyphenyl]-3,5-hexadien-2-one with a yield of over 98%.
[0064] The experimental data are: 1H NMR (400MHz, dmso) δ9.83(s, 1H), 7.42–7.29(m, 2H), 7.00(d, J=15.5Hz, 1H), 6.89(d, J=10.6Hz, 1H), 6.77–6.72(m, 2H), 6.13(d, J=15.5Hz, 1H), 2.21(s, 3H).
[0065] 2) Preparation of 6-[4-phenylbenzyloxy]-3,5-hexadien-2-one (compound 3)
[0066] Mix 5g of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one, 5ml tetrahydrofuran, 3g potassium carbonate and 3.8g benzyl bromide, stir at reflux at 70°C for 3h, add water to quench, and the column layer The p-benzyloxycinnamaldehyde was obtained by analysis, and the yield reached more than 95%.
[0067] The experimental data is: 1 H NMR (400MHz, dmso) δ7.51 (d, J = 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com