A diradical compound composed of triarylmethyl radical and nitroxide radical and its salt, its preparation method and application
A technology of triarylmethyl and nitroxide radicals, applied in the field of their preparation, diradical compounds and their salts, can solve problems such as no room for improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A diradical compound composed of triarylmethyl radicals and nitroxide radicals and salts thereof described in this embodiment has a structural formula as follows:
[0052]
[0053] The preparation steps of compound 1 are as follows formula 11:
[0054]
[0055]
[0056] One, the preparation of compound A4:
[0057]
[0058] Two, the preparation of compound 1:
[0059] 1. Dissolve compound A1 (1-1.5eq) and HOBt (2-5eq) in redistilled DCM, stir, add DIPEA (3-10eq), BOP (0.9-1.5eq) first dissolve in redistilled DCM, then Add the reaction solution, activate for 0.5-2h, then dissolve compound A2 (0.9-2eq) in redistilled DCM, add the reaction solution, and react at room temperature for 0.5-18h. Treatment: extract with DCM and water, successively wash with citric acid, saturated sodium carbonate solution, saturated brine, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, separate with normal phase silica gel column, mobile phase: ethy...
Embodiment 2
[0068] A diradical compound composed of triarylmethyl radicals and nitroxide radicals and salts thereof described in this embodiment has a structural formula as follows:
[0069]
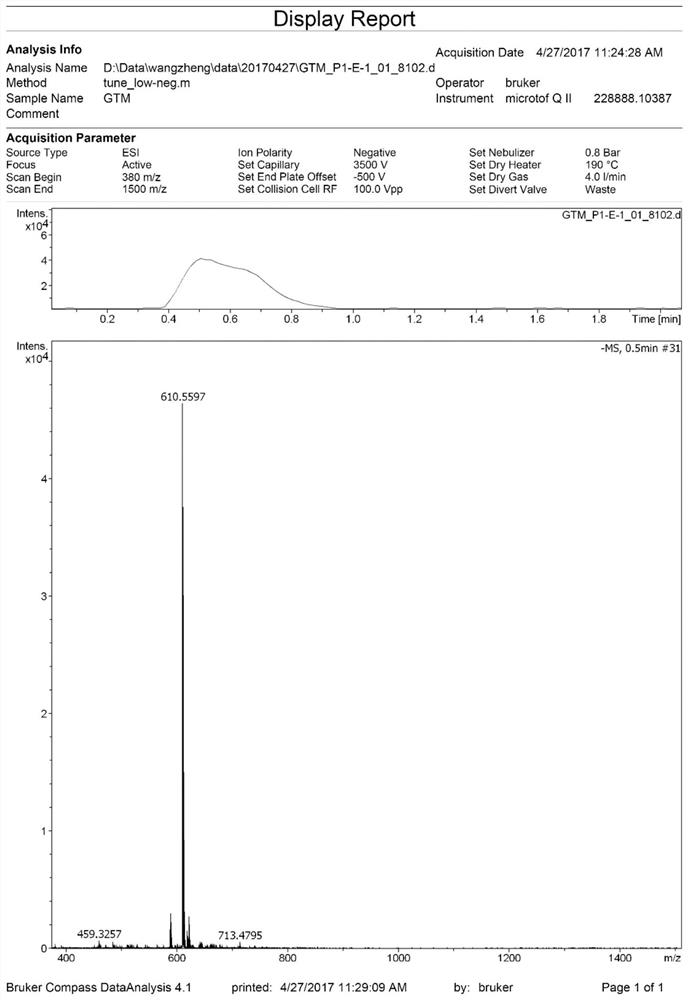
[0070] 1. The preparation steps of compound 2 are the same as the method in Example 1.
[0071] In this example, compound A1 is Commercially available.
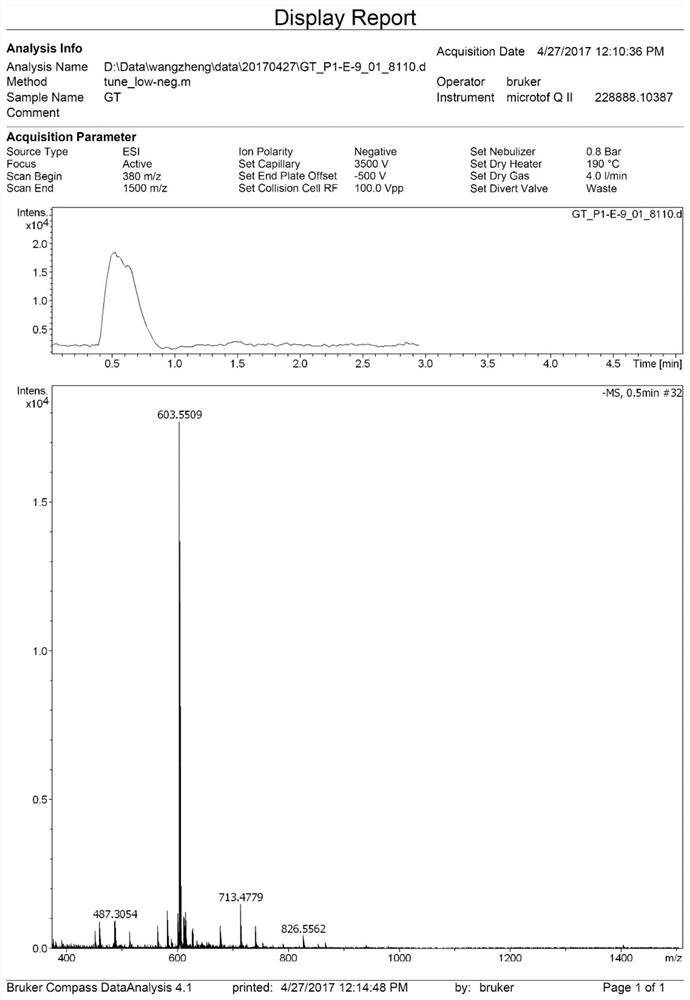
[0072] Such as figure 2 It is the high-resolution mass spectrometry data of compound 2 in this example
[0073] 2. DNP enhancement effect experiment of compound 2
[0074] The experimental method of DNP enhanced performance test is the same as that of compound 1 test.
[0075] The DNP enhancement factor of compound 2 was 76. Compared with compound 1, the enhancement factor of DNP has increased by 33%, and the improvement effect is remarkable.
Embodiment 3
[0077] A diradical compound composed of triarylmethyl radicals and nitroxide radicals and salts thereof described in this embodiment has a structural formula as follows:
[0078]
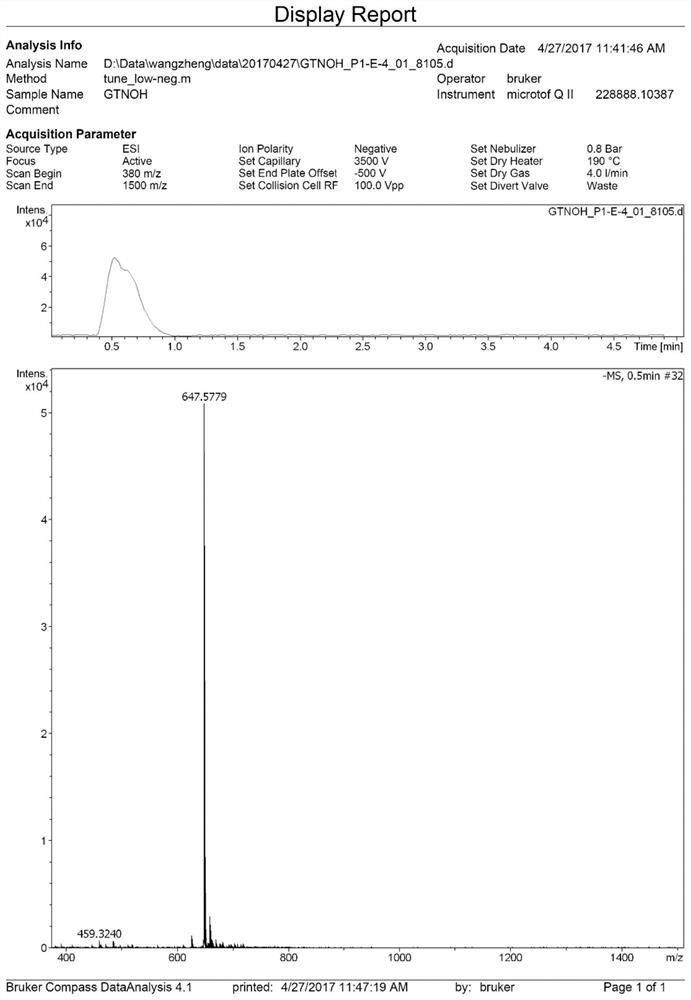
[0079] The preparation steps of compound 3 are as follows formula 13:
[0080]
[0081] One, the preparation of compound 3:
[0082] Diethylene glycol amine (0.5-2eq), imidazole (2-5eq), DMAP (0.05-0.5eq), tert-butyldimethylsilyl chloride (0.5-2eq) were placed in the reaction flask, argon protection, ice Bath, add anhydrous DCM, stir at zero degree for half an hour, then transfer to room temperature and stir for 2-18 hours to complete the reaction. Post-reaction treatment: wash with saturated brine and evaporate to dryness to obtain the pure product 6 as a colorless transparent oil with a yield of 75-95%.
[0083] The product obtained in the previous step (0.9-2eq) and nitroxide radical (1eq) were dissolved in anhydrous THF, protected by argon, stirred at room temperature for 2 hours, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com