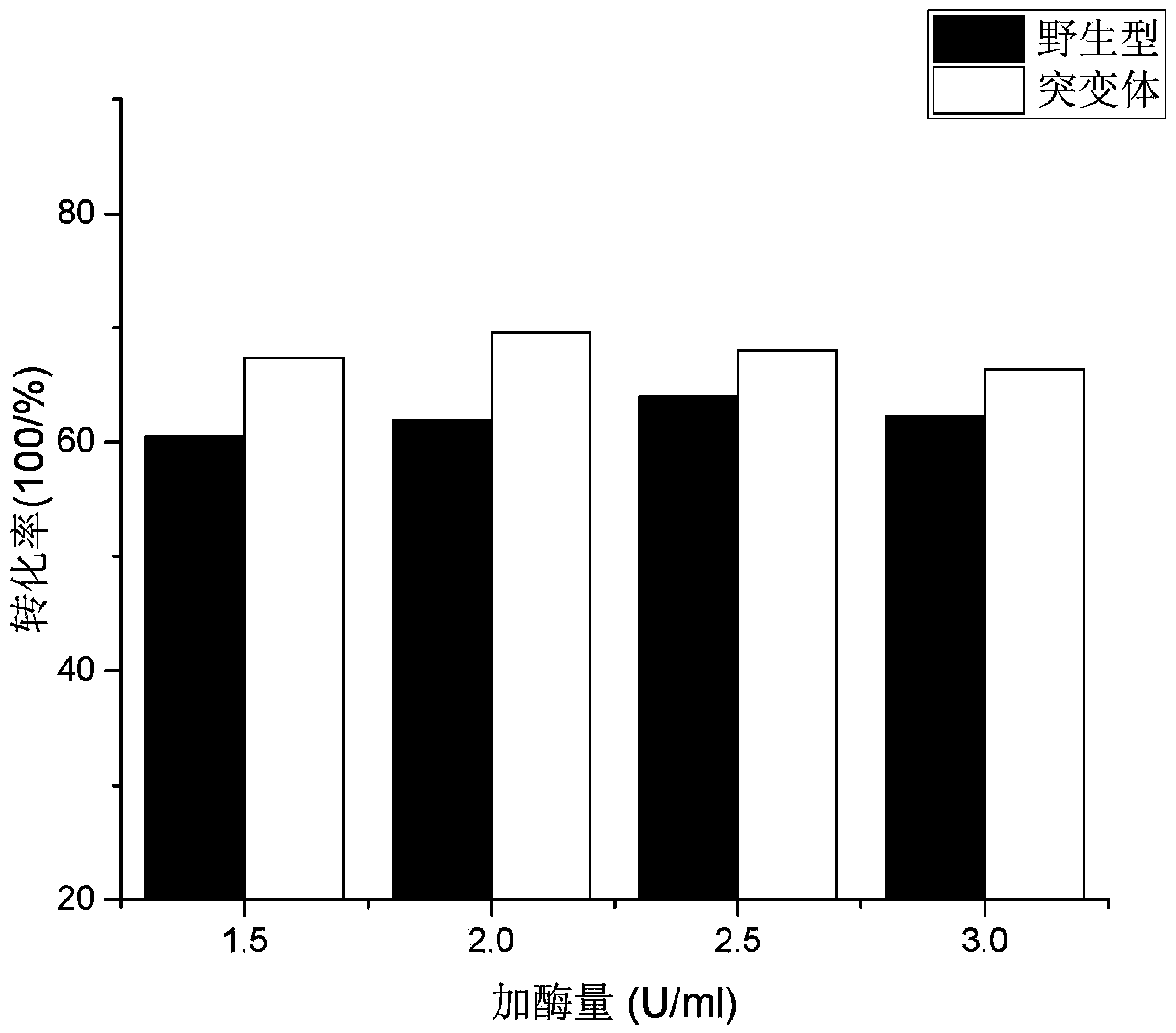
Thermal stability and trehalose yield improved MTSase mutant
A technology with improved yield and thermal stability, which is applied in the fields of enzyme engineering and protein engineering, can solve the problems of poor enzyme stability at medium temperature, high cost, and high conversion temperature, etc., so as to reduce the amount of enzyme added, improve stability, and produce trehalose The effect of increased conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Expression of wild-type maltooligosaccharyl trehalose synthase
[0044] The Arthrobacter ramosus genome was used as a template, and the nucleotide sequence was SEQ ID NO.4: the forward primer of CATATGCCAGCTTCTACATAT and the nucleotide sequence was SEQ ID NO.5: the reverse primer of ATTACTGGTTGAAACCTAAAAGCTT was used to clone the target gene treY containing the coding sequence, after After HindIII and NdeI double digestion, it was ligated with the expression vector pET24a, transformed into Escherichia coli BL21(DE3), and treY / pET24a / BL21(DE3) was obtained, and treY / pET24a / BL21(DE3) was inoculated in LB liquid medium (containing 100mg / L Kanamycin) was grown for 10 h, and the seeds were inserted into TB liquid fermentation medium (containing 100 mg / L Kanamycin) according to the inoculum size of 5%, and after Escherichia coli engineering bacteria were cultivated at 37° C. for 2 h, 0.01 mmol / L L final concentration of IPTG (isopropylthio-β-D-galactoside) to indu...
Embodiment 2
[0045] Example 2: Preparation and expression of a single mutant of maltooligosaccharyl trehalose synthase of the present invention
[0046] (1) Construction of single mutants S44P, L26F, T413Y:
[0047] Site-directed mutagenesis: Using PCR technology, using the treY / pET-24a(+) plasmid as a template, the mutation primers are:
[0048] S44P:
[0049] The nucleotide sequence of the forward primer is SEQ ID NO.6: CCTCTGTTAGAAAGTGAA CCA GGTTCTTCAC (the mutation site is underlined)
[0050] The nucleotide sequence of the reverse primer is SEQ ID NO.7: GTGAAGAACC TGG TTCACTTTCTAACAGAGG (underline is the mutation site)
[0051] L26F:
[0052] The nucleotide sequence of the forward primer is SEQ ID NO.8: CAGCCCGTATTGTTCCATAT TTT CATCGTTTAGGC (the mutation site is underlined)
[0053] The nucleotide sequence of the reverse primer is SEQ ID NO.9: GCCTAAACGATG AAA ATATGGAACAATACGGGCTG (the mutation site is underlined)
[0054] T413Y:
[0055] The nucleotide sequence of the for...
Embodiment 3
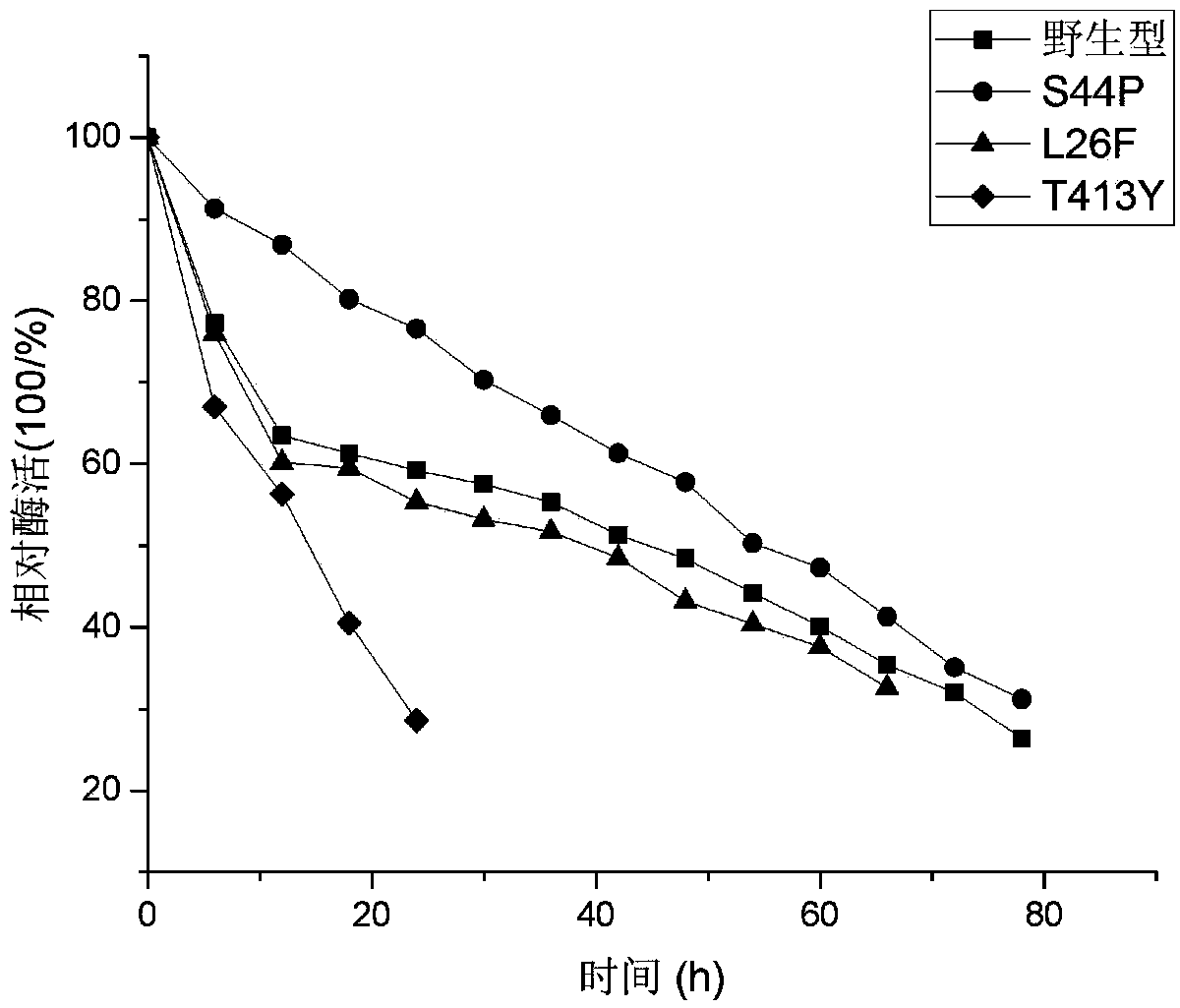
[0062] Example 3: Thermal stability analysis of the maltooligosaccharide-based trehalose synthase mutant of the present invention
[0063] The fermented intracellular crude enzyme liquid obtained in Example 1 and Example 2 was purified, and the stability of the purified enzyme was tested.
[0064] Test results such as figure 1 As shown, it can be seen that the half-lives of mutant S44P, L26F, T413Y and wild type are 55h, 40h, 15h and 43h respectively, the mutant S44P is 12h higher than the wild type, which is 1.3 times that of the wild type, and the half-life of the mutant L26F and T413Y is longer than the wild type Type reduction, respectively reduced by 3h, 28h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com