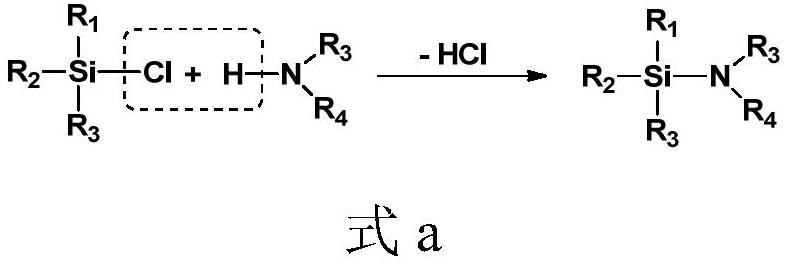
A six-membered nitrogen-oxygen-silicon heterocyclic compound and its preparation method
A technology of heterocyclic compound and silicon oxynitride, which is applied in the field of six-membered silicon oxynitride heterocyclic compound and its preparation, can solve the problem of weak reducibility of hydrogen-containing silane, achieve short reaction time, good application prospects, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Preparation of 6-tert-butyl-2((phenylimino)-4-methylphenol
[0034] Under the protection of nitrogen, use 2mmol 2-tert-butyl-p-methyl-phenol and 2.2mmol paraformaldehyde to react in acetonitrile (100mL) at reflux (100℃~110℃) for 2 hours, cool to room temperature, add 5% Wash with hydrochloric acid, then extract with diethyl ether (3x100mL), collect the diethyl ether phase, then wash with saturated brine (100mL), and wash the organic phase with anhydrous MgSO 4 Dry and filter. Concentrated and recrystallized to obtain 2-tert-butyl-p-methyl-valylal with a yield of 95%.
[0035] 1 mmol of 2-tert-butyl-p-methyl-valylal and 1.2 mmol of 2,6-diisopropylaniline synthesized above were refluxed (90° C.) in absolute ethanol (50 mL) and reacted for 1 hour. Cool to room temperature and recrystallize from ethanol to obtain 6-tert-butyl-2(2,6-diisopropylbenimino)-4-methylphenol with a yield of 90%.
[0036]2) Preparation of 3,4-benzyl, 2-oxa, 6-aza, 1,1-dichlorosilane compounds ...
Embodiment 2
[0048] 1 mmol of 2-tert-butyl-p-methyl-glycanal and 1.2 mmol of aniline synthesized according to the route of Example 1 were refluxed (90° C.) in absolute ethanol (50 mL) and reacted for 1 hour. Cool to room temperature, recrystallize with ethanol to obtain 6-tert-butyl-2((phenylimino)-4-methylphenol, the yield is 90%, and set aside.
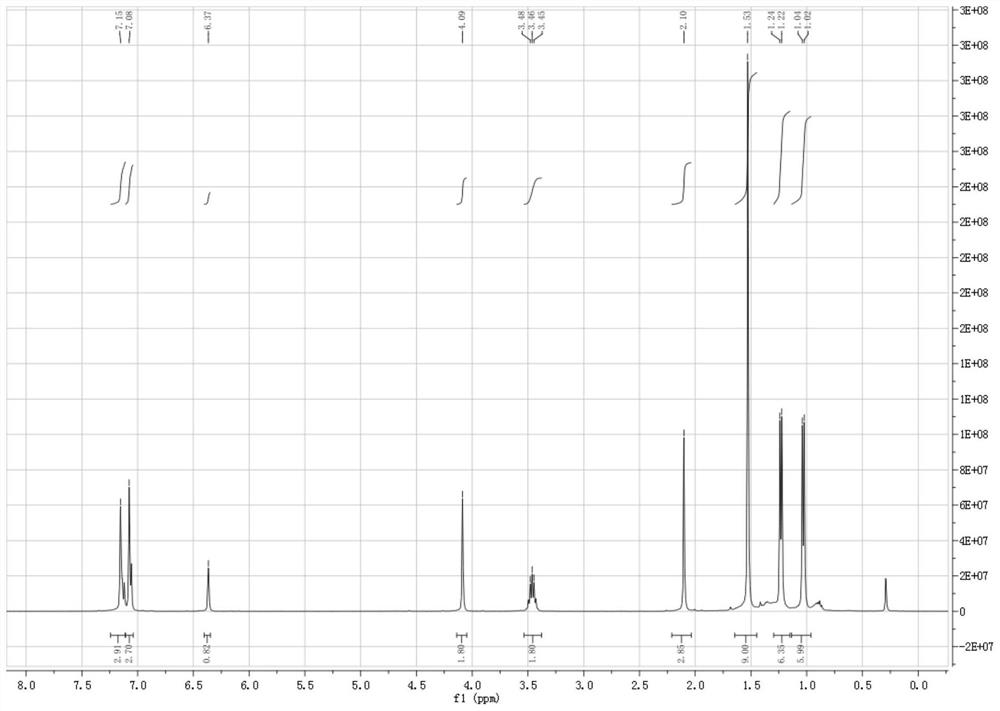
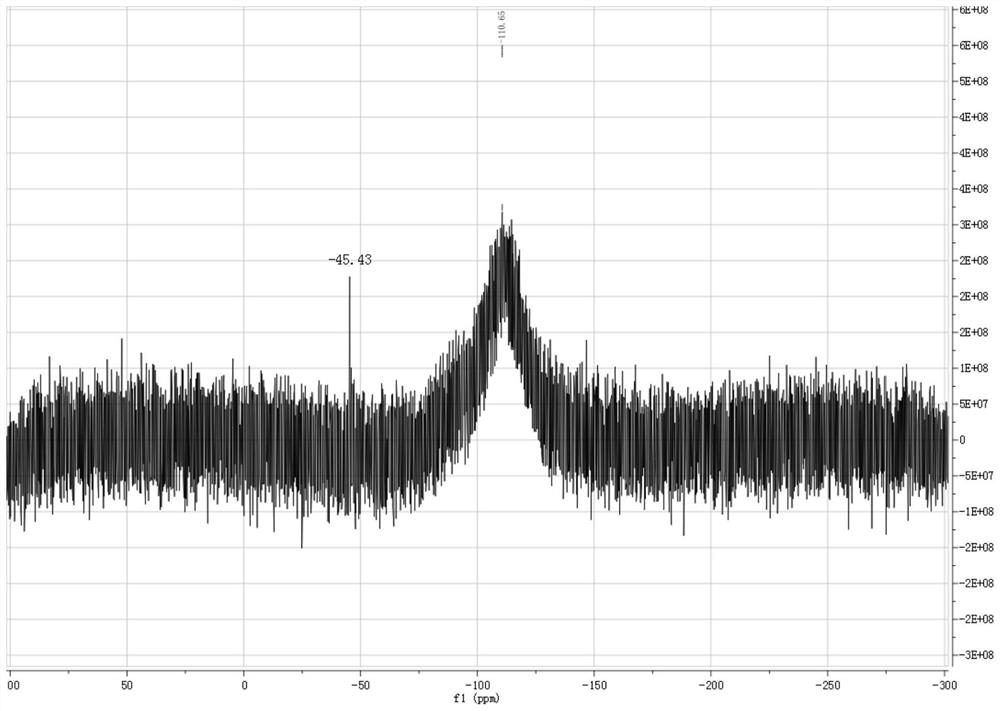
[0049] Under nitrogen protection, 50 mL of n-hexane was added to a dry Shrek bottle, and then 1.2 mmol of trichlorosilane was added to dissolve 1.0 mmol of 6-tert-butyl-2((phenylimino)- 4-Methylphenol and 1.1mmol of triethylamine were slowly added dropwise to the above system at 0°C, reacted for 1.5h, concentrated the solvent to 10mL, placed in a -20°C refrigerator for recrystallization, and obtained white crystal 3, 4-Benzene, 2-oxa, 6-aza, 1,1-dichlorosilane compounds, the yield is 95%, and the structural formula is as follows:
[0050]
Embodiment 3
[0054] 1 mmol of 2-tert-butyl-p-methyl-glycanal and 1.2 mmol of pentafluoroaniline synthesized according to the route of Example 1 were refluxed (90° C.) in absolute ethanol (50 mL) and reacted for 1 hour. Cool to room temperature and recrystallize with ethanol to obtain 6-tert-butyl-2((pentafluorophenylimino)-4-methylphenol with a yield of 92%, which is ready for use.
[0055] Under the protection of nitrogen, 50 mL of n-hexane was added to a dry Shrek bottle, and then 1.2 mmol of trichlorosilyl hydrogen was added to dissolve 1.0 mmol of 6-tert-butyl-2((pentafluorobenimino) in 25 mL of n-hexane )-4-Methylphenol and 1.1mmol of triethylamine were slowly added dropwise to the above system at 0°C, reacted for 1.5h, concentrated the solvent to 10mL, placed in a -20°C refrigerator for recrystallization, and obtained light yellow crystals , 3,4-benzyl, 2-oxa, 6-aza, 1,1-dichlorosilane compounds, the yield is 95%, and the structural formula is as follows:
[0056]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap