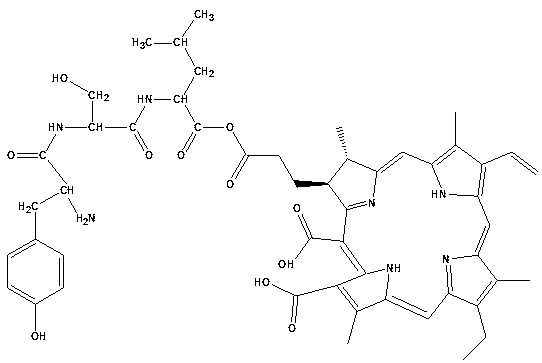
Tyroserleutide-chlorin e6 monoester and preparation method thereof
A technique of chlorin and leupepide, which is applied in the field of tyroserupin-chlorin e6 monoester, and can solve the problems of tumor growth inhibition, no reports on tyroserupin and chlorin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
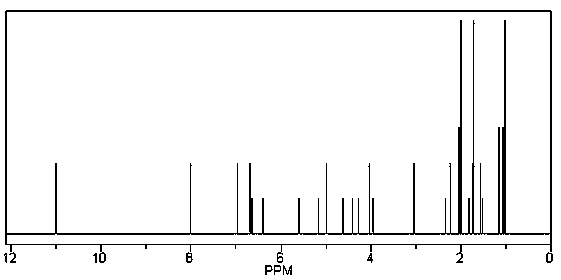
[0024] Chlorin e6 (Chlorin e6, Ce6) is dissolved in ethanol under the condition of protective gas and dark, and it is A. After tyroserleutide was dissolved in glacial acetic acid, A was added, and the reaction was carried out under stirring at 36°C for 28 hours; wherein chlorin e6 (Chlorin e6, Ce6) and tyroserleutide were in equimolar amounts.
[0025] After centrifugation, the supernatant was dialyzed against deionized water and freeze-dried.
[0026] The dry matter is separated by silica gel column chromatography, and the eluent is a mixed solution of dichloromethane / methanol with a volume ratio of 3 / 7 to 7 / 3;
[0027] After dichloromethane / methanol is evaporated, dissolve with methanol or acetonitrile, refine and purify with reverse phase column chromatography, the eluent is methanol / water mixed solution or acetonitrile / water mixed solution; the filler used in the reversed phase column is C 18 ; The volume ratio of the methanol / water mixed solution or the acetonitrile / wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com