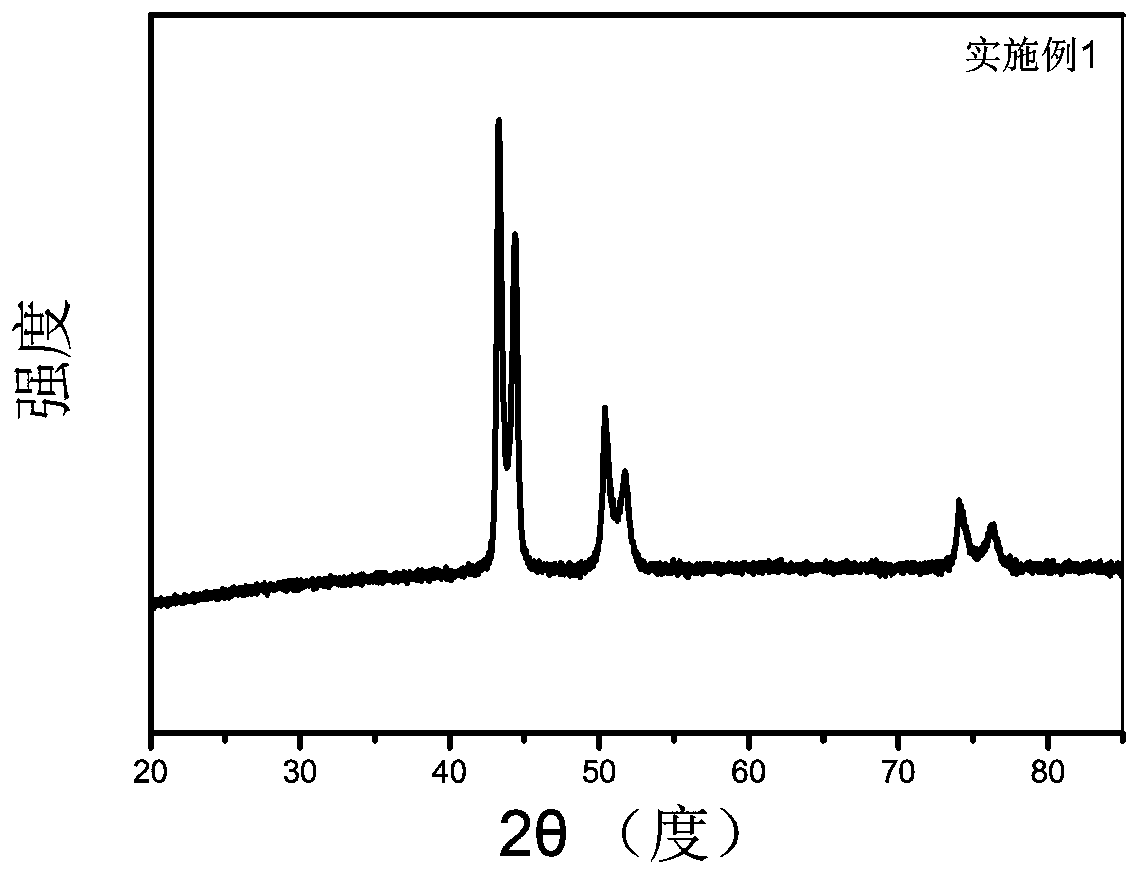
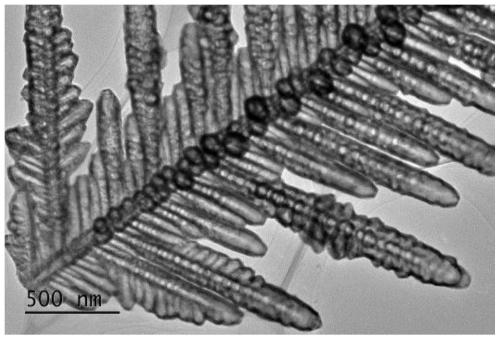
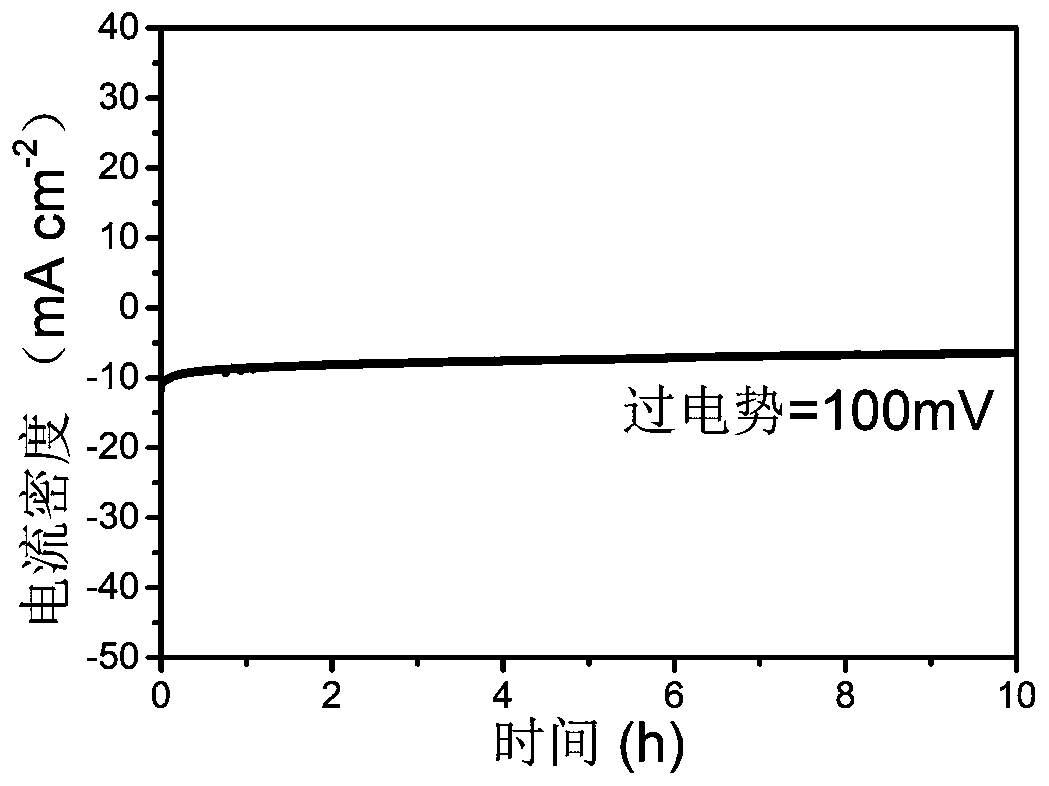
A kind of nickel phosphorus copper electrocatalyst and preparation method thereof
A technology of catalyst and copper electricity, which is applied in the field of materials, can solve the problems of large-scale production limitations, reduce the overpotential of hydrogen evolution, and limited availability, and achieve the best surface structure and performance, non-toxic and harmless physical and chemical properties, and enhanced The effect of catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of Nickel Phosphorus Copper Electrocatalyst
[0030] (1) Pretreatment of electrode materials: use carbon rods as anodes, nickel sheets as cathodes, use 20% hydrochloric acid to clean the nickel sheets in an ultrasonic cleaner for 30 minutes to remove the surface oxide layer, then wash with deionized water, and dry spare;
[0031] (2) Electrolyte preparation: Pipette 100mL concentration of 0.2M nickel chloride, 100mL concentration of 0.2M sodium phosphate, 100mL concentration of 0.25M ammonium chloride and 100mL concentration of 0.02M copper chloride with a pipette gun Stir the solution into a glass beaker with a magnetic stirrer for 30 minutes, and stir until the flocs in the solution are evenly suspended in the solution to obtain an electrolyte;
[0032] (3) Deposition of nickel-phosphorus-copper electrocatalyst: In the electrolyte solution prepared in step (2), put the electrode material in step (1), and use IT6874A DC power supply to set a constant curre...
Embodiment 2
[0042] Preparation of Nickel Phosphorus Copper Electrocatalyst
[0043](1) Pretreatment of electrode materials: use carbon rods as anodes, nickel sheets as cathodes, use 20% hydrochloric acid to clean the nickel sheets in an ultrasonic cleaner for 30 minutes to remove the surface oxide layer, then wash with deionized water, and dry spare;
[0044] (2) Preparation of electrolyte solution: Pipette 300mL concentration of 0.2M nickel chloride, 300mL concentration of 0.2M sodium phosphate, 300mL concentration of 0.25M ammonium chloride and 300mL concentration of 0.01M copper chloride with a pipette gun Stir the solution into a glass beaker with a magnetic stirrer for 30 minutes, and stir until the flocs in the solution are evenly suspended in the solution to obtain an electrolyte;
[0045] (3) Deposition of nickel-phosphorus-copper electrocatalyst: In the electrolyte solution prepared in step (2), put the electrode material in step (1), and use IT6874A DC power supply to set a con...
Embodiment 3
[0051] Preparation of Nickel Phosphorus Copper Electrocatalyst
[0052] (1) Pretreatment of electrode materials: use carbon rods as anodes, nickel sheets as cathodes, use 20% hydrochloric acid to clean the nickel sheets in an ultrasonic cleaner for 30 minutes to remove the surface oxide layer, then wash with deionized water, and dry spare;
[0053] (2) Preparation of electrolyte: pipette 450mL concentration of 0.2M nickel chloride, 450mL concentration of 0.2M sodium phosphate, 450mL concentration of 0.25M ammonium chloride and 450mL concentration of 0.03M copper chloride with a pipette gun Stir the solution into a glass beaker with a magnetic stirrer for 30 minutes, and stir until the flocs in the solution are evenly suspended in the solution to obtain an electrolyte;
[0054] (3) Deposition of nickel-phosphorus-copper electrocatalyst: In the electrolyte solution prepared in step (2), put the electrode material in step (1), and use IT6874A DC power supply to set a constant cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com