A mini protein reactor for proteome sample preparation and its application
A mini-protein and proteomics technology, applied in the field of mini-protein reactors, can solve the problems of undetectable and pancreatic cancer treatment failure, and achieve the effects of short time-consuming, high sensitivity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
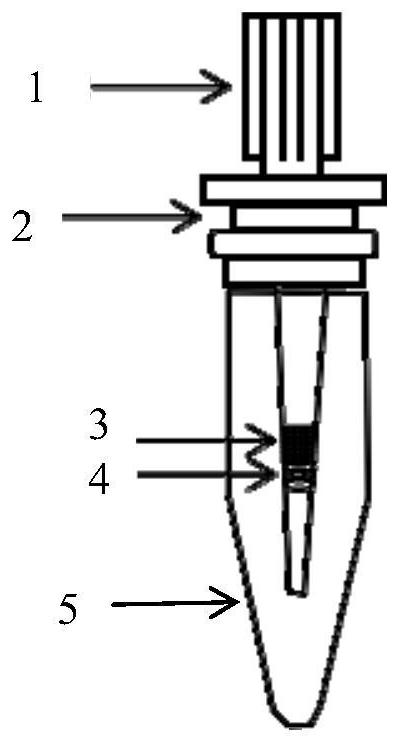
[0054] A mini protein reactor used in proteome sample preparation, called SPMC, its structure diagram is shown in figure 1 shown. The mini protein reactor includes pipette tips (1), adapters (2), IMAC magnetic beads (3), C 18 beads (C 18 small column) (4), Eppendorf tube (Eppendorf centrifuge tube) (5); pipette tip (1) is provided with several C successively from bottom to top 18 beads (4), several IMAC magnetic beads (3); C 18 The beads (4) are in contact with the IMAC magnetic beads (3); the adapter (2) is sleeved on the pipette tip (1), and the adapter (2) is placed on the upper end of the Eppendorf tube (5); the pipette tip (1) ) into the Eppendorf tube (5).
[0055] The pipette tip is preferably a 10-200 μL pipette tip;
[0056] The Eppendorf tube is preferably a 500μL-2.0mL Eppendorf tube;
[0057] The C 18 The beads are preferably 2~5μm Empore C 18 beads; more preferably 3 μm Empore C 18 beads;
[0058] The IMAC magnetic beads are preferably 10-50 μm IMAC magn...
Embodiment 2
[0061] The present invention is to utilize the method for preparing proteomics sample of SPMC mini-protein reactor, comprises the following steps successively:
[0062] (1) Collection of serum samples: After collecting PDAC patient serum and healthy human serum, store them in a -80°C refrigerator.
[0063] (2) Design of SPMC: such as figure 1 As shown, SPMC is achieved by combining several C 18 beads (3 μm Empore, USA) were loaded into a standard 200 μL pipette tip, and then a certain amount of 20 μm POROS IMAC magnetic beads (Applied Biosystems, USA) was introduced. Then place the SPMC tip through the adapter into a 2.0 mL Eppendorf tube. Calculation of C based on the amount of protein 18 The number of beads and the amount of IMAC magnetic beads, for example: 0.4-1.5 mg IMAC can be used for the separation of 2-20 μg protein (binding capacity of about 30 μg protein / mg beads).
[0064] (3) Enrichment: Before sample loading, several C 18 200 μL pipette tips of beads, severa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com