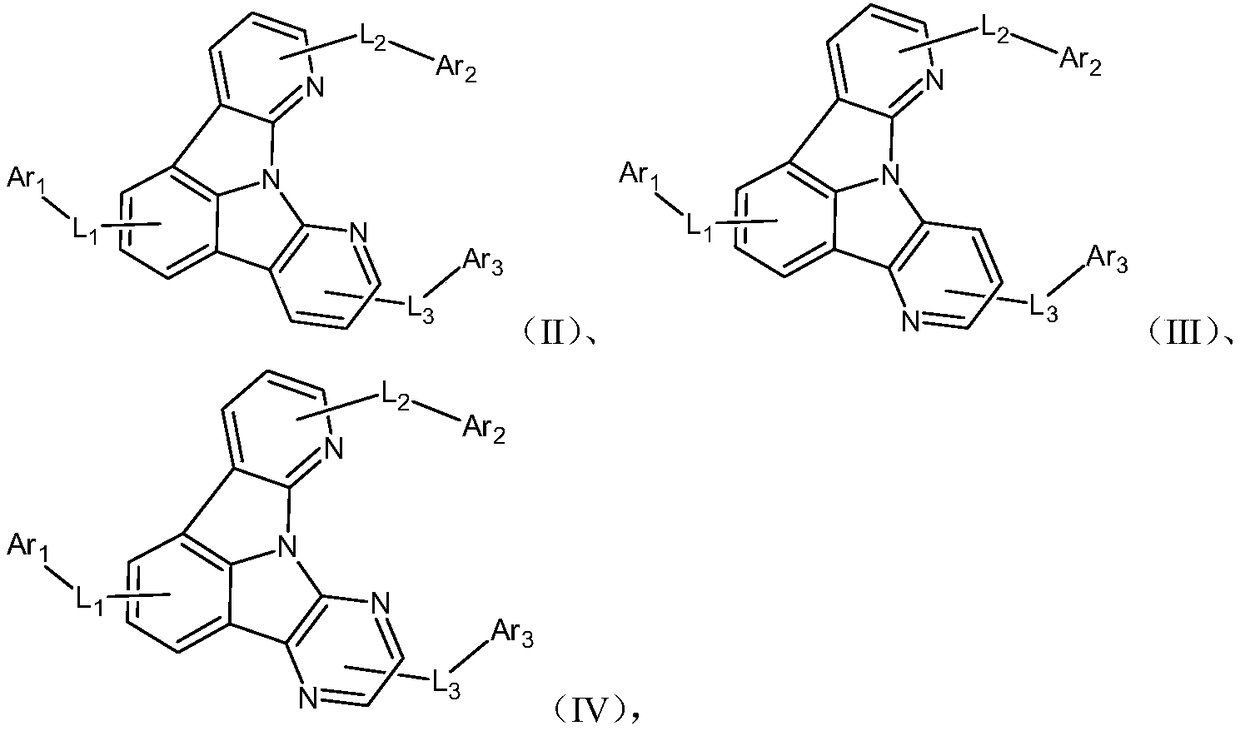
Heterocyclic nitrogen containing organic light-emitting material and application thereof
A technology of luminescent materials and nitrogen heterocyclic rings, which is applied in luminescent materials, organic chemistry, compounds of group 4/14 elements of the periodic table, etc., can solve problems such as insufficient heat resistance, and achieve material stability, easy sublimation processability, Effect of Charge Injection Balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the synthesis of compound 3a:
[0064] Compound 3a was prepared according to the following chemical synthesis route, and a white powder material was obtained. 0.1 g of compound 3a was completely dissolved in 40 ml of dichloromethane. The measured PL of the fluorescence spectrum was 399 nm. The molecular structure was verified by mass spectrometry as shown in Table 3.
[0065]
[0066] (1) Synthesis of Compound 5:
[0067] Compound 4 (12.1g, 50mmol) was completely dissolved in 100ml of DMF, and NBS (8.9g, 50mmol) was added in batches and reacted at 0°C for 10h. After the reaction was completed, the reaction solution was poured into 1L of water. Precipitate, filter, rinse the solid twice with water and ethanol respectively, collect the solid and vacuum-dry it to obtain compound 5 (13.8 g, 40.9 mmol, yield 81.9%).
[0068] (2) Synthesis of compound 6:
[0069] The dried compound 5 (6.9g, 20.4mmol) was dissolved in 100ml of tetrahydrofuran, cooled to -78°...
Embodiment 2
[0074] Embodiment 2: the synthesis of compound 20a:
[0075] Compound 20a was prepared according to the following chemical synthesis route, and a white powder material was obtained. 0.1 gram of compound 20a was completely dissolved in 80 ml of dichloromethane, and the measured fluorescence spectrum was PL=407nm. The molecular structure was verified by mass spectrometry as shown in Table 3.
[0076]
[0077] (1) Synthesis of Compound 8:
[0078] Compound 4 (12.1g, 50mmol) was completely dissolved in 100ml of DMF, and NBS (17.8g, 100mmol) was added in batches and reacted at 0°C for 10h. After the reaction was completed, the reaction solution was poured into 1L of water. Precipitate, filter, rinse the solid twice with water and ethanol respectively, collect the solid and vacuum-dry it to obtain compound 8 (16.1 g, 40.4 mmol, yield 85.2%).
[0079] (2) Synthesis of compound BIK-2B:
[0080] The dried compound 8 (7.96g, 20mmol) was dissolved in 100ml of tetrahydrofuran, cooled...
Embodiment 3
[0085] Embodiment 3: Synthetic preparation of other compounds
[0086] Similarly, according to the above principles of synthetic chemistry, following a certain mass ratio of substances, and under the experimental conditions of compound 3a and compound 20a, the following main materials were synthesized, and the specific listed compounds were verified by mass spectrometry. Fragments, see Table 1 below for details:
[0087] Table 1: Compound synthesis and characterization
[0088]
[0089]
[0090]
[0091]
[0092]
[0093]
[0094]
[0095]
[0096] Example of performance evaluation
[0097] Examples of OLED devices
[0098] The light-emitting device provided by the present invention comprises a first electrode, a hole transport layer, a light-emitting layer, an electron transport layer and a second electrode deposited sequentially. Wherein the hole transport layer, the light-emitting layer, and the electron transport layer are all organic layers, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com