Sustained-release high-efficiency poly-gamma-glutamic acid/dodecyl dimethyl benzyl ammonium chloride nano-bactericide as well as preparation method and application thereof
A technology of dodecyl dimethyl benzyl ammonium chloride and glutamic acid, which is applied in the medical field, can solve problems such as slow release, and achieve excellent biocompatibility, good slow release performance, and good antibacterial and inhibitory properties. The effect of bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
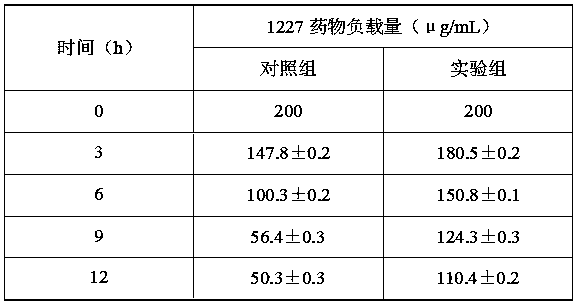
[0028] Preparation of nano-antibacterial agent under the condition of poly-γ-glutamic acid / chitooligosaccharide mass ratio of 10:1
[0029] The preparation of step 1 novel high-efficiency nano antibacterial agent
[0030] 1) In the 8.75 mL system, prepare a poly-γ-glutamic acid / chitooligosaccharide solution with a mass ratio of 10:1 (total concentration: 5 mg / mL) (the solvent is deionized water, the same below), and stir evenly. Prepared into poly-γ-glutamic acid / chitooligosaccharide matrix solution;
[0031] 2) EDC and NHS were prepared into 0.2 M concentration solutions respectively, and mixed at a ratio of 2:1 to obtain an EDC / NHS solution. Take 1.25 mL of the prepared EDC / NHS solution and add it to the prepared matrix solution, and stir at room temperature for 5 h, after forming a homogeneous solution, ultrasonically crush for 5 h to prepare loaded nanoparticles;
[0032] 3) Prepare 10 mL of 1227 solution with a concentration of 400 μg / mL, add it to the prepared nanopart...
Embodiment 2
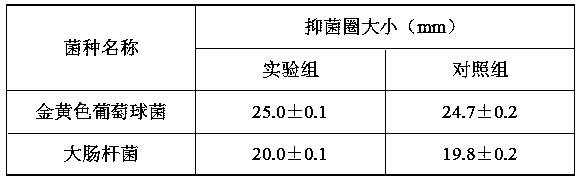
[0043] Example 2 Preparation of nano-antibacterial agent under the condition of 12:1 mass ratio of poly-γ-glutamic acid / oligochitosan
[0044] The preparation of step 1 novel high-efficiency nano antibacterial agent
[0045] 1) In an 8.75 mL system, prepare a poly-γ-glutamic acid / chitooligosaccharide solution with a mass ratio of 12:1 (total concentration: 5 mg / mL), stir evenly, and prepare a poly-γ-glutamic acid / shell Oligosaccharide matrix solution;
[0046] 2) EDC and NHS were prepared into 0.2 M concentration solutions respectively, and mixed at a ratio of 2:1 to obtain an EDC / NHS solution. Take 1.25 mL of the prepared cross-linking agent EDC / NHS solution and add it to the prepared matrix solution. , stirred for 5 h to form a homogeneous solution, and ultrasonically crushed for 5 h to prepare loaded nanoparticles;
[0047] 3) Prepare 10 mL of 1227 solution with a concentration of 400 μg / mL, add it to the prepared nanoparticle solution at a ratio of 1:1, and continue to s...
Embodiment 3
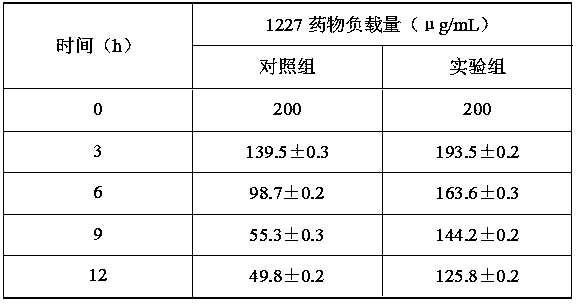
[0058] Example 3 Preparation of nano-antibacterial agent under the condition that the mass ratio of poly-γ-glutamic acid / oligochitosaccharide is 14:1
[0059] The preparation of step 1 novel high-efficiency nano antibacterial agent
[0060] 1) In an 8.75 mL system, prepare a poly-γ-glutamic acid / chitooligosaccharide solution with a mass ratio of 14:1 (total concentration: 5 mg / mL), stir evenly, and prepare a poly-γ-glutamic acid / shell Oligosaccharide matrix solution;
[0061] 2) EDC and NHS were prepared into 0.2 M concentration solutions respectively, and mixed at a ratio of 2:1 to obtain an EDC / NHS solution. Take 1.25 mL of the prepared cross-linking agent EDC / NHS solution and add it to the prepared matrix solution. , stirred for 5 h to form a homogeneous solution, and ultrasonically crushed for 5 h to prepare loaded nanoparticles;
[0062] 3) Prepare 10 mL of 1227 solution with a concentration of 400 μg / mL, add it to the prepared nanoparticle solution at a ratio of 1:1, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| load ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com