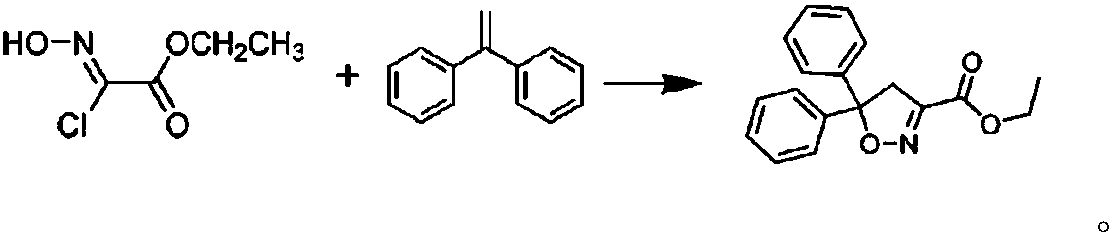
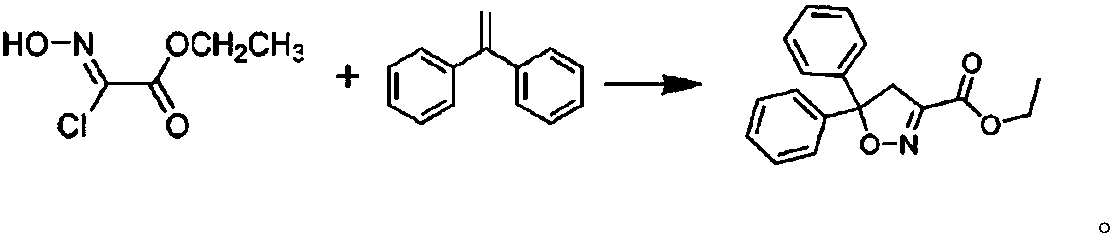
Synthesizing method of isoxadifen-ethyl for industrialized production
A technology of isoxadifen and its synthesis method, which is applied in the field of production of isoxadifen, can solve the problems of by-products and difficulties, and achieve the effect of low cost and high purity of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 (laboratory stage):
[0025] Preparation of phenylmagnesium chloride Grignard reagent: add 4.5g (0.19mol) magnesium chips, 65ml THF, add 0.45g bromoethane and raise the temperature to 65°C, add dropwise 18.7g (about 0.17mol) chlorobenzene, 65ml THF, Keep warm for 4h-5h, lower the temperature to 15°C, and after GC detects that the chlorobenzene reaction is complete, lower the temperature to 15°C to prepare phenylmagnesium chloride Grignard reagent (1), with a content of 98.9%, which is directly used in the next reaction.
[0026] Preparation of 1,1-stilbene: Control the temperature at 10°C-15°C, slowly drop the mixture of 14g (0.12mol) acetophenone and 30ml toluene into the phenylmagnesium chloride Grignard reagent, keep it warm for 30min after dropping, Stop the reaction and set aside. Then add the aqueous solution prepared by adding 6g ammonium chloride and 23g water thereto, then add 60ml toluene, stir for 30 minutes, and adjust pH=1-3 with hydrochloric ...
Embodiment 2
[0029] Embodiment 2 (industrial scale-up experiment):
[0030] Raw material type:
[0031] Raw material 1, magnesium scrap, tetrahydrofuran; the mass ratio of magnesium scrap to tetrahydrofuran is about 1:2.98, of which magnesium scrap is 45kg;
[0032] Raw material 2, chlorobenzene and tetrahydrofuran; the mass ratio of chlorobenzene and tetrahydrofuran is about 1:1.02, of which chlorobenzene is 187kg;
[0033] Raw material 3, acetophenone, toluene; ethyl bromide; the mass ratio of acetophenone, toluene and ethyl bromide is about 31:6.2:1, of which ethyl bromide is 4.5kg;
[0034] In industrial application, the above feeding amount can be converted according to 50kg of magnesium chips.
[0035] Specific implementation steps:
[0036] The 2000L first reaction kettle is first filled with toluene to bring water to anhydrous, then put in raw material 1, add bromoethane at 40°C-50°C, heat up to 65°C and reflux, then drop in raw material 2, add dropwise for about 8-10h, after dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com