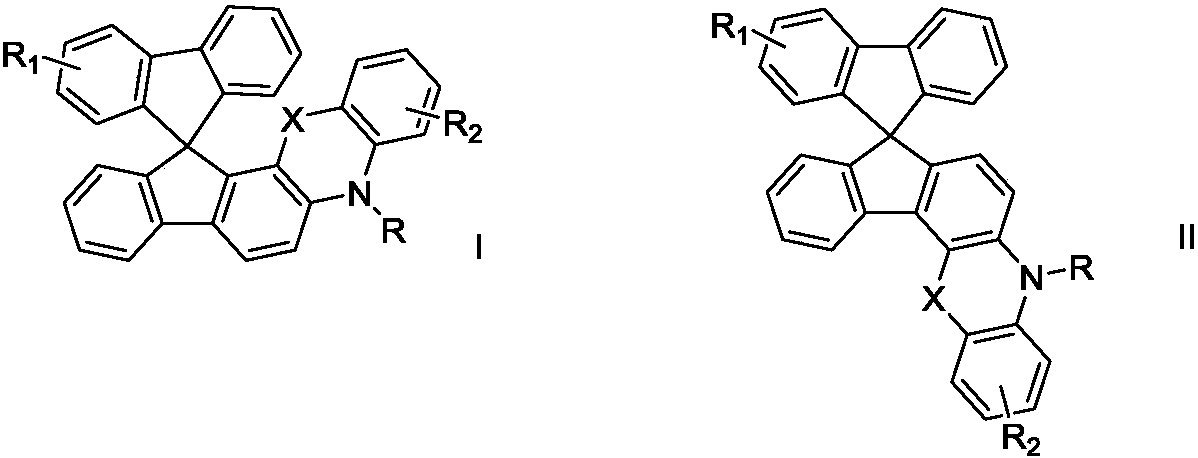
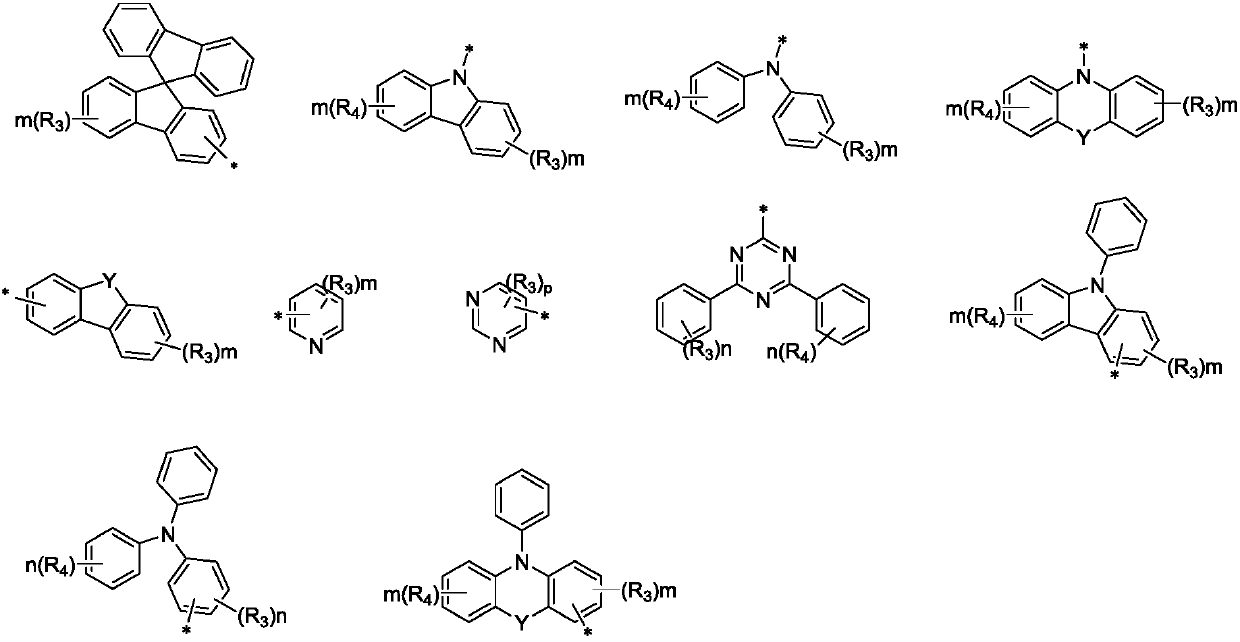
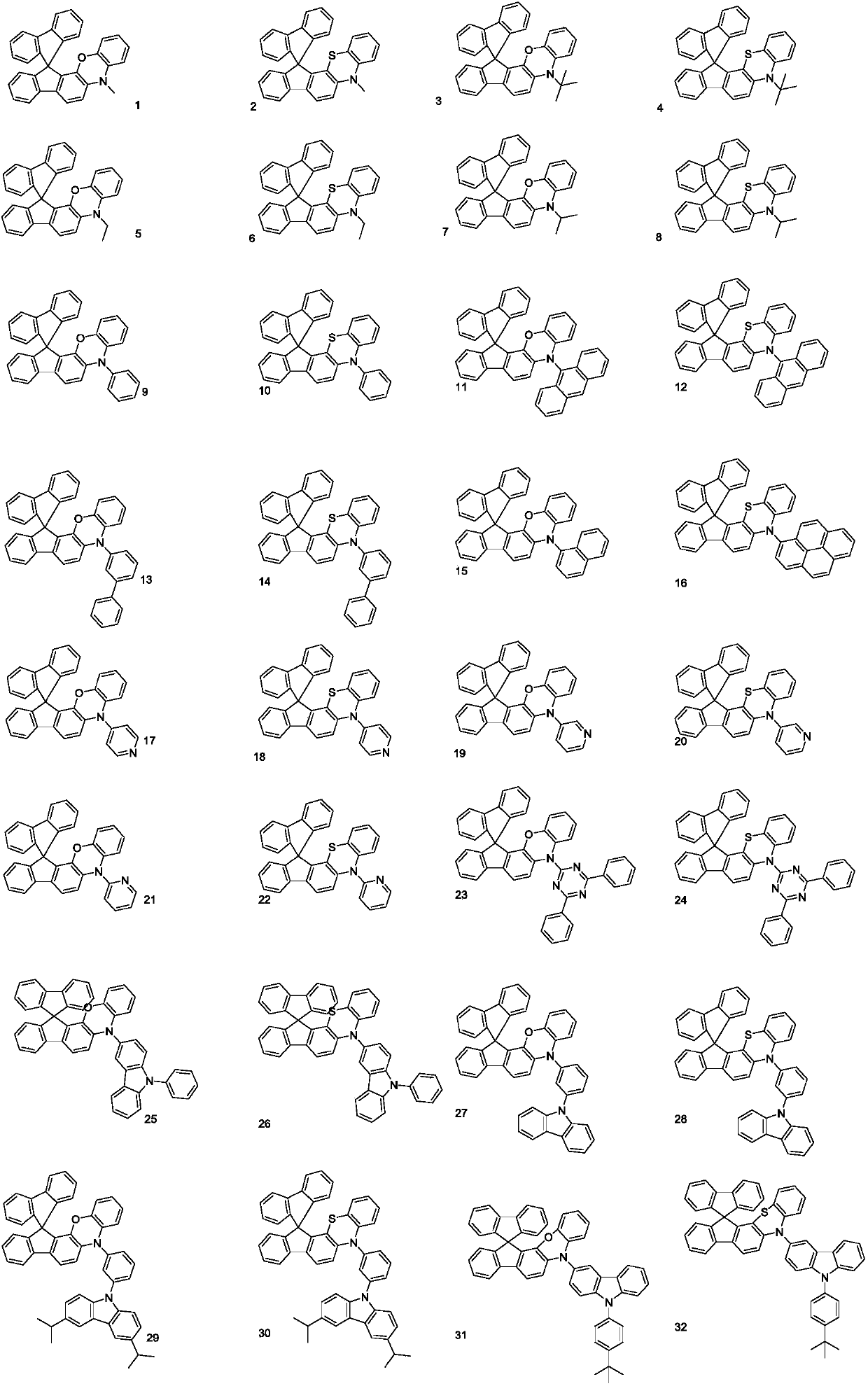
Spirofluorene and nitrogen-containing hetercyclic organic electroluminescent material and organic luminescent device thereof
A nitrogen heterocycle and electroluminescence technology, applied in luminescent materials, electro-solid devices, electrical components, etc., can solve the problems of low luminous efficiency and low lifespan, and achieve high luminous efficiency, high glass transition temperature, carrier The effect of transmission balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of compound 9
[0071]
[0072] Step1. Under the protection of nitrogen, 100mmol of 2-bromobiphenyl was dissolved in anhydrous THF, and added dropwise to a round bottom flask filled with 110mmol of magnesium chips. After triggering, continue to drop 2-bromobiphenyl solution, and reflux for 3 hours. , add dropwise 9-1, 120mmol THF solution to the reaction system, reflux overnight after dropwise addition, cool and filter the yellow solid, add it to aqueous ammonium chloride solution and stir for 2 hours, filter and dry to obtain a white solid, put the white solid into boiling in ethanol solution, cooled, poured into water, a white solid was precipitated, filtered, recrystallized from ethanol to obtain the product, 90mmol 9-2.
[0073] Step2. Add 90mmol of 9-2 to a three-necked flask, dissolve in acetic acid solution, react at 90°C for 12 hours, pour into water, filter, and recrystallize from toluene to obtain 70mmol of product 9-3.
[007...
Embodiment 2
[0077] Embodiment 2: the preparation of compound 20
[0078] With embodiment 1. Replace o-nitrophenol in step3. with o-nitrothiophenol. Replace iodobenzene in Step5. with 3-iodopyridine.
Embodiment 3
[0079] Embodiment 3: the preparation of compound 23
[0080] Same as Example 1, the iodobenzene in Step5. was replaced by 2-chloro-4,6-diphenyl-1,3,5-triazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com