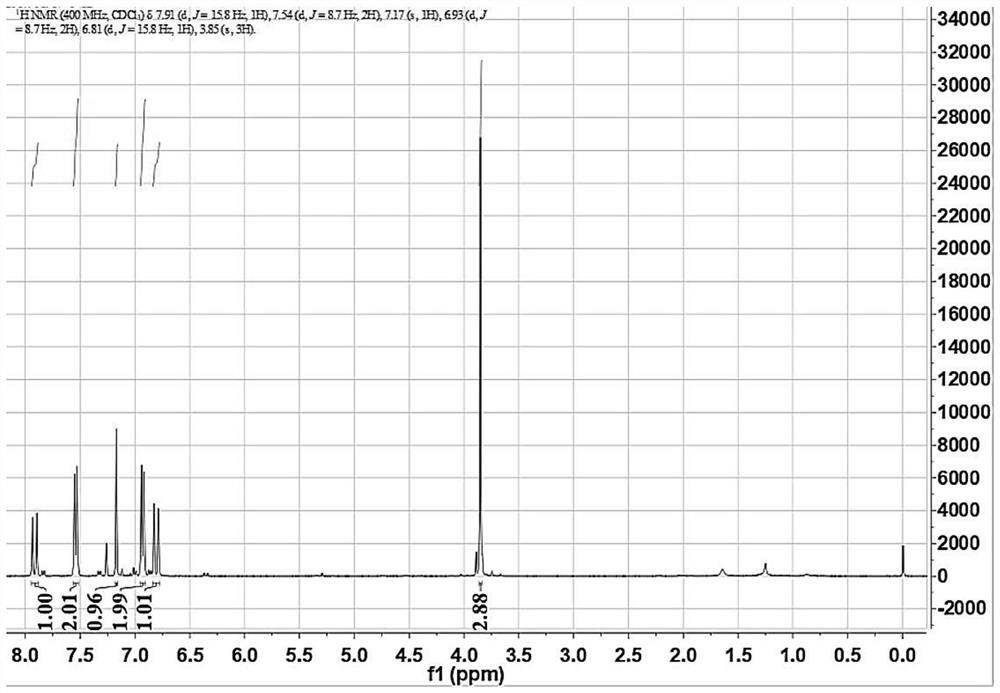
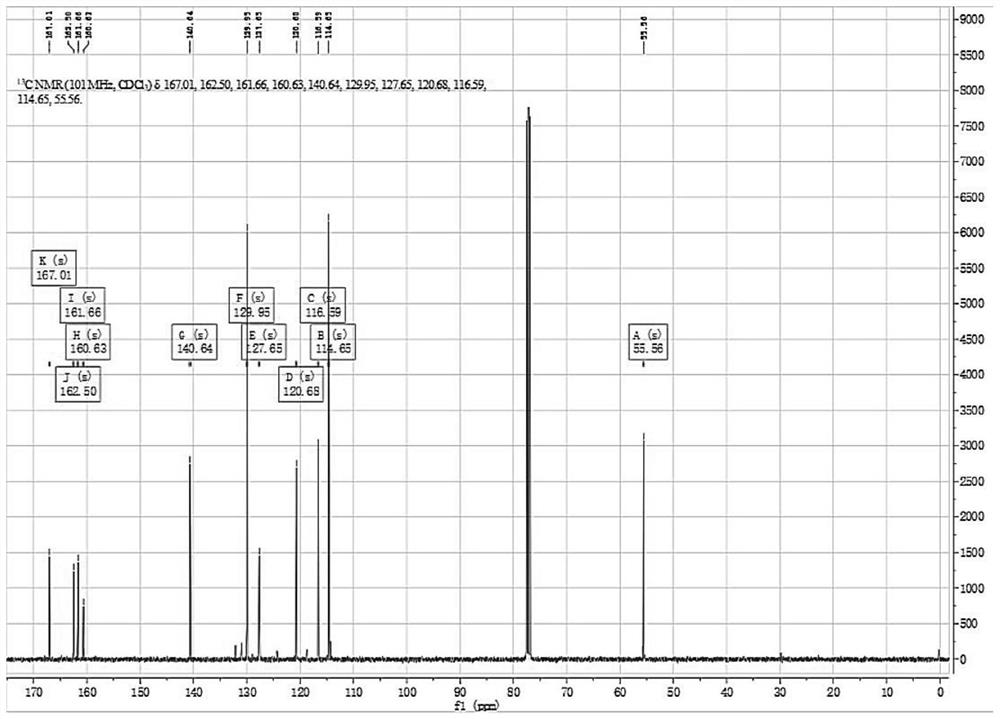
Purification method of 2,4-dichloro-6-styryl pyrimidines
A purification method, pyrimidine-based technology, applied in the direction of organic chemistry, can solve the problems of large solvent consumables, poor separation effect, consumption, etc., and achieve the effect of simple operation, high conversion rate, and simplified operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Preparation method
[0029] 1) Take by weighing 12.0g sodium hydride that adopts 60% mineral oil to disperse, join in the round-bottomed flask, and feed nitrogen to fully discharge the air in the round-bottomed flask;
[0030] 2) Add 300mL tetrahydrofuran to the round bottom flask, stir at 450prm; then, weigh 22.0g 2,4-dichloro-6-methylpyrimidine, dissolve it in 50mL tetrahydrofuran and add it to the round bottom flask;
[0031] 3) Measure 15mL of p-methoxybenzaldehyde into a round-bottomed flask and react for 8 hours; cool the round-bottomed flask in an ice-water bath for 10 minutes, and add a small amount of water dropwise until no bubbles are generated;
[0032] 4) Take the reaction product in the reaction system, add 400mL of dichloromethane and 400mL of water for liquid separation and extraction, repeat the operation three times, and combine the organic phase; dry the organic phase with anhydrous magnesium sulfate, filter to obtain anhydrous organic phase, organ...
Embodiment 2
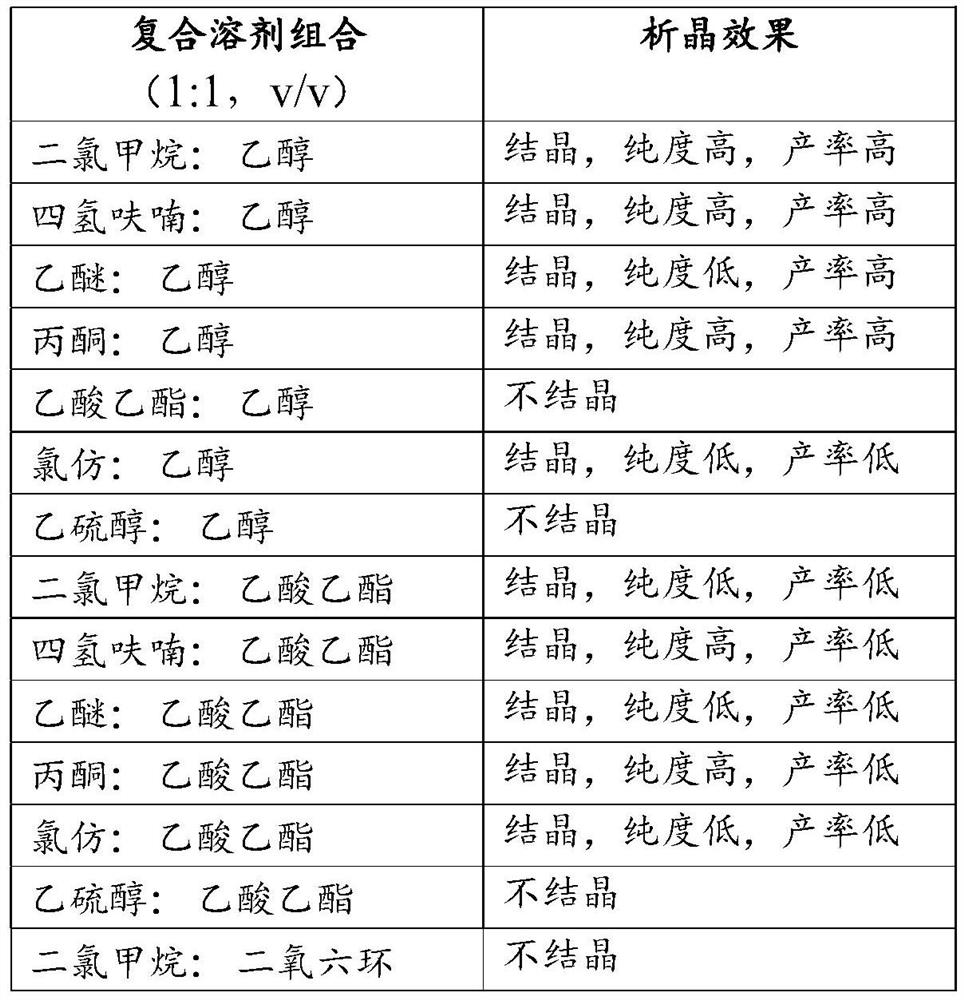
[0042] In this embodiment, multiple sets of composite solvents are used to replace the dichloromethane-ethanol (1:1, v / v) in Step 2 of Example 1, and the crystallization effect is shown in Table 1:
[0043] Table 1
[0044]
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com