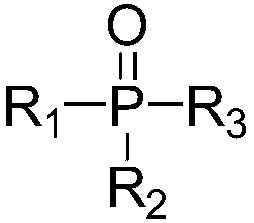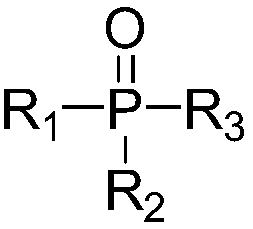Synthetic method for 4,6-dichloropyrimidine
A technology of dichloropyrimidine and a synthesis method, applied in directions such as organic chemistry, can solve the problems of large amount of catalyst, high cost, cumbersome recovery and reuse process of organic bases, etc., and achieves avoiding recovery and reuse, low cost, and catalyst amount. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 212g of triphosgene (content 99%, 0.71mol) was dissolved in 500mL of nitrobenzene for use.
[0029] In the device equipped with reflux condenser, thermometer, stirrer and constant pressure dropping funnel, add 4,6-dihydroxypyrimidine (114g, content 98%, 1mol), triphenylphosphine oxide (8.4g, content 99 %, 0.03mol), cobalt phthalocyanine (0.57g, 0.001mol), stir and mix evenly, heat up to 90-95°C, and add dropwise a nitrobenzene solution of triphosgene to react.
[0030] Sampling and analysis after 5 hours of reaction, HPLC analysis showed that the content of 4,6-dihydroxypyrimidine was 0.25%, and the content of 4,6-dichloropyrimidine was 98.2%. degree-0.095Mpa), to obtain 4,6-dichloropyrimidine 143.8g (content 99.7%), yield 96.2% (based on 4,6-dihydroxypyrimidine).
Embodiment 2
[0040]In a device equipped with a reflux condenser, a thermometer, a stirrer and a constant pressure dropping funnel, add 4,6-dihydroxypyrimidine (114g, content 98%, 1mol), tri-n-octylphosphine oxide (7.74g, content 99%, 0.02mol), cobalt phthalocyanine (0.46g, 0.0008mol), stir and mix evenly, heat up to 110-120°C, add 297g of diphosgene (content 99%, 1.5mol) dropwise for reaction.
[0041] Sampling and analysis after 6 hours of reaction, HPLC analysis showed that the content of 4,6-dihydroxypyrimidine was 0.31%, and the content of 4,6-dichloropyrimidine was 97.5%. Degree -0.095Mpa), to obtain 4,6-dichloropyrimidine 141.6g (content 99.8%), yield 94.8% (calculated as 4,6-dihydroxypyrimidine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com