In-vitro expression and separation and purification method for chemotactic factor TARC/CCL17
A chemokine and external recombination technology, applied in the field of molecular biology, can solve problems such as complex evaluation methods and SCORAD not widely used, and achieve the effect of reducing production costs and production difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
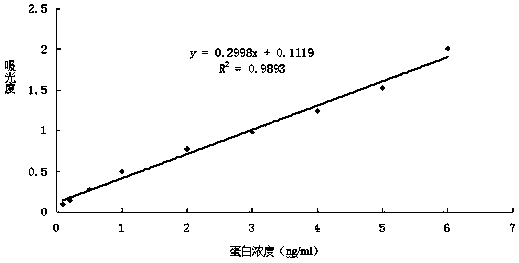
Image
Examples
Embodiment Construction
[0015] The present invention is further explained below in conjunction with the examples, but the examples do not limit the present invention in any form.
[0016] 1. Integrate the known cDNA sequence (SEQ ID NO:1) corresponding to TARC / CCL17 into the E. coli vector PETM3C, and transform the constructed vector into E. coli BL21.
[0017] 2. Cultivate the bacteria in a shaker at 37°C and 200rad / min until OD 600 At 0.6-0.8, add IPTG to make the concentration of IPTG in the bacterial solution reach 0.2mmol / L, and place it in a shaker at 37°C and 200rad / min for 16h.
[0018] 3. After centrifuging at 4000g for 20min to collect the bacteria, suspend them in a small amount of solution containing 50mM Tris-HCl and 20mM NaCl.
[0019] 4. After ultrasonically disrupting the cells, centrifuge at 18000g for 20min, and collect the supernatant.
[0020] 5. Use affinity chromatography to purify the protein, the binding solution is 50mM Tris-HCl, 20mM NaCl, 5mM imidazole, the washing soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com