Application of 23-hydroxybetulinic acid in preparation of medicines for preventing and treating influenza
A technology of betulinic acid and hydroxyl group, applied in the field of preparation of 23-hydroxy betulinic acid in the preparation of influenza prevention and treatment drugs, can solve the problems of no patent disclosure, no report on the anti-influenza virus application of 23-hydroxy betulinic acid, etc., and the preparation process is mature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
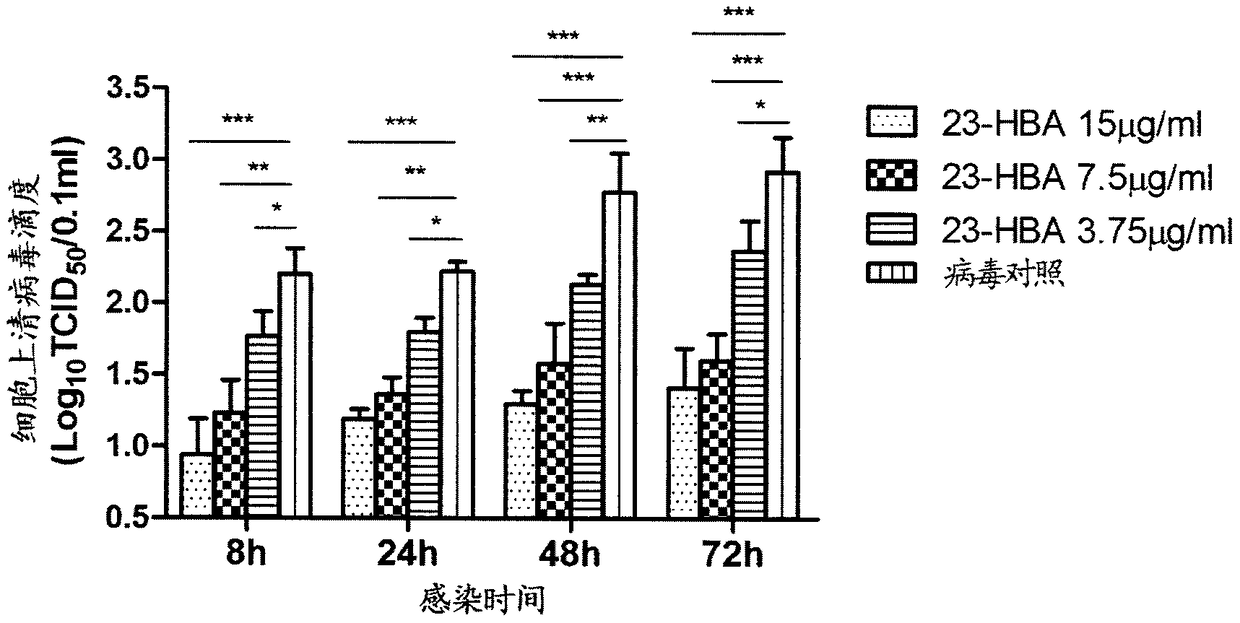
[0024] Example 1: Inhibitory Effect of 23-Hydroxybetulinic Acid on Proliferation of H1N1 Subtype Influenza Virus in A549 Cells
[0025] After the A549 cells grow to a single layer, add the H1N1 strain virus liquid (containing 100 TCID50) diluted with the basal medium, 100 μL / well, incubate at 37°C for 2 hours, discard the virus supernatant, and phosphate buffered saline (PBS solution) ) was washed once, and 3.75, 7.5 and 15 μg / ml of 23-hydroxybetulinic acid were added respectively, 100 μL / well. In the experiment, a normal control group (without adding test drug, without adding influenza virus liquid) and an influenza virus control group (without adding test drug) was set up at the same time, and each test concentration was set in three parallels. The cells continued to be cultured, and the culture was terminated at 8h, 24h, 48h, and 72h respectively. After observing the pathological changes, the cell plate was repeatedly frozen and thawed three times at -80°C and 4°C, so that ...
Embodiment 2
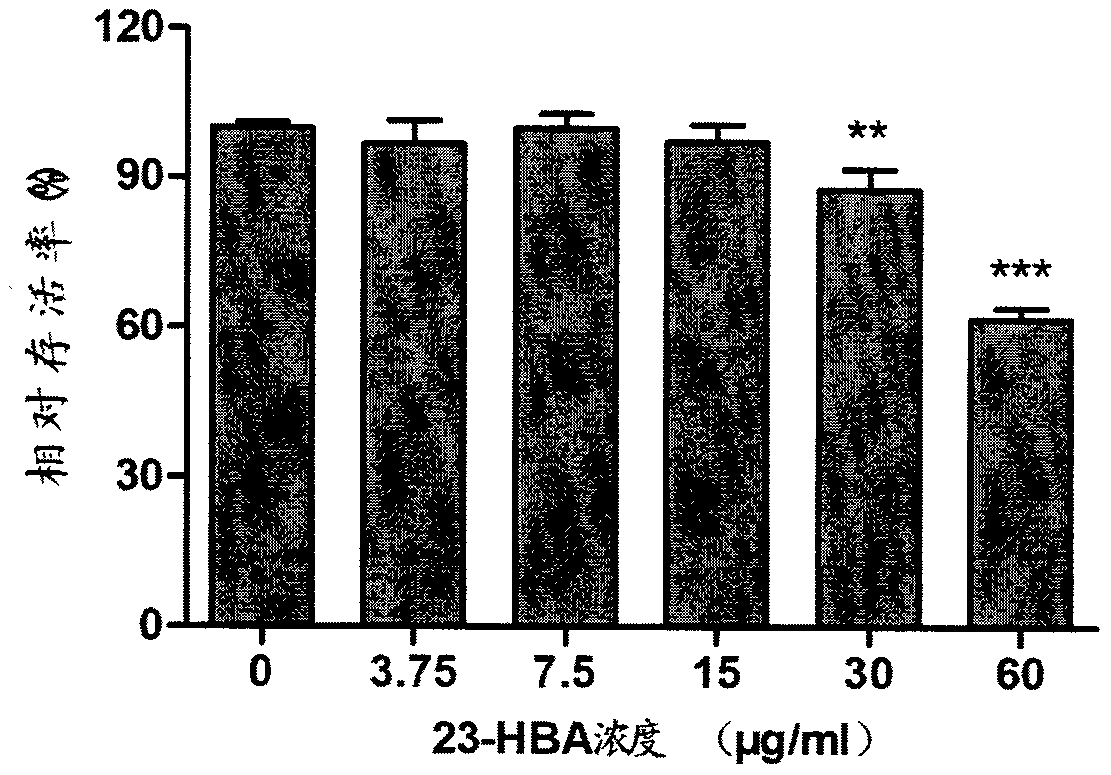
[0027] Embodiment 2: The test of 23-hydroxy betulinic acid to A549 cytotoxicity
[0028] After the A549 cells grow to a single layer in the 96-well cell culture plate, the medium is discarded, washed twice with PBS (phosphate buffered saline), and 100 μL of 23-hydroxybetulinic acid diluted with different concentrations in DMEM cell maintenance solution is added , six replicate wells were made for each concentration. Place at 37°C, 5% CO 2 Incubate for 48 hours in an ambient incubator protected from light. Discard 20 μl of supernatant, add 20 μl of MTT solution to each blank, and continue to culture in the cell culture incubator for 4 hours. Terminate the culture, carefully absorb the culture medium in the well, and discard it. 150 μl of dimethyl sulfoxide (DMSO) was injected into each well, and the shaker was shaken at a low speed for 10 min to fully dissolve the formazan crystals. Absorbance was measured at 490 nm using an enzyme-linked immunosorbent assay. from figure...
Embodiment 3
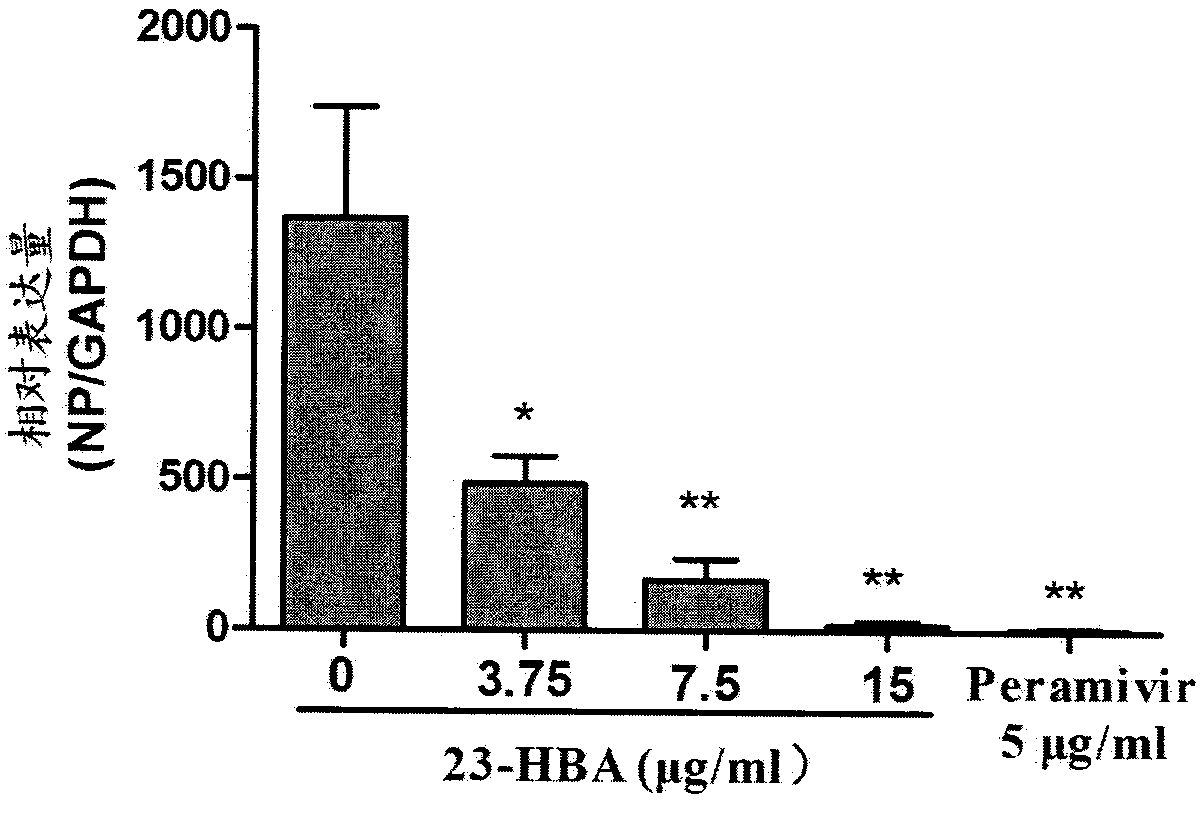
[0029] Example 3: Inhibitory Effect of 23-Hydroxybetulinic Acid on Influenza Virus RNA Proliferation in A549 Cells
[0030] After the A549 cells grow to a monolayer in the 6-well cell culture plate, discard the medium, wash with PBS (phosphate buffer saline) twice, and add 100TCID diluted with DMEM cell maintenance solution. 50H5N1 virus, 1ml / well, incubated at 37°C for 2h, discarded the virus supernatant, washed 2 times with PBS, and added 15, 7.5, and 3.75 μg / mL of 23-hydroxybetulinic acid, 2mL / well, respectively. At the same time, a normal control group (no test drug, no H5N1) and H5N1 control group (no test drug) were set up in the experiment, and three parallels were set for each test concentration. The cells were cultured at 37°C until 48 h after infection. After observing the lesions, freeze and thaw the cell plate at -80°C and 4°C for three times to fully lyse the cells, so that all the viruses in the cells are released into the cell supernatant, and then the supernat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com