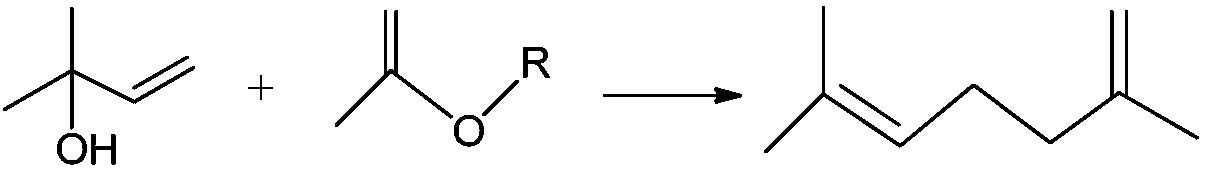Method for synthesizing methyl heptenone from 2-methyl-3-buten-2-ol
A technology for synthesizing methylheptenone and alcohol, which is applied in the direction of condensation to prepare carbonyl compounds, organic chemistry, etc., can solve the problems of product quality decline, easy deterioration, harsh storage conditions, etc., and achieve the effect of reducing three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
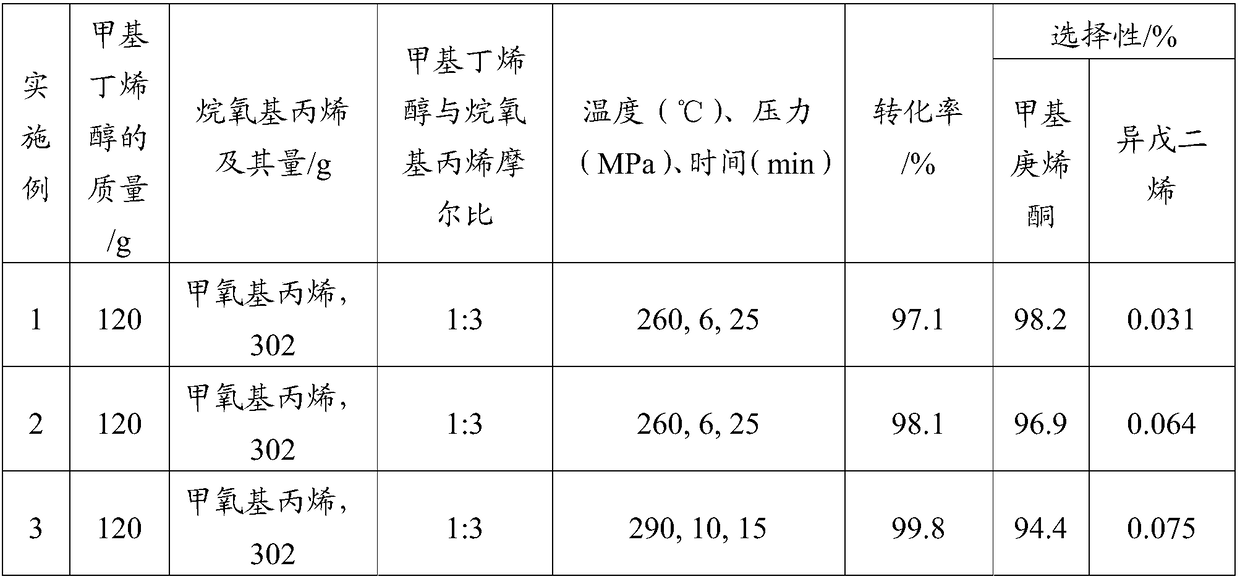
[0038] Under normal temperature and pressure operating conditions, first add 120g of 2-methyl-3-buten-2-ol and 302g of 2-methoxypropene to the autoclave, pressurize to 6MPa, and heat to 260°C, When the near-critical state of 2-methyl-3-buten-2-ol is reached, the Saucy-Marbet reaction is performed for 25 minutes, and then the temperature of the reaction liquid is lowered to normal temperature and pressure. The reaction liquid was distilled under normal pressure to remove and recover the light components of 2-methoxypropene and 2,2-dimethoxypropane, and distill under reduced pressure to obtain 167.64 g of a colorless and transparent liquid product.
[0039] The product obtained was confirmed to be methyl heptenone by gas mass spectrometry. The reaction liquid was measured by gas chromatography internal standard method, and the conversion rate of the raw material 2-methyl-3-buten-2-ol was 97.1%. The selectivity of methylheptenone is 98.2%, and the selectivity to by-product isoprene ...
Embodiment 2
[0041] Under normal temperature and pressure operating conditions, first add 120g of 2-methyl-3-buten-2-ol to the autoclave, pressurize and heat it to make 2-methyl-3-buten-2-ol reach Near the critical state, then, 302 g of 2-methoxypropylene was pumped into the autoclave by a high pressure liquid phase pump, and 167.13 g of a colorless and transparent liquid product was prepared in the same manner as in Example 1.
[0042] The product was confirmed to be methylheptenone by gas mass spectrometry, and the same gas chromatography internal standard method as in Example 1 was used for measurement. The conversion rate of the raw material 2-methyl-3-buten-2-ol was 98.1%. , The selectivity to the product methylheptenone was 96.9%, and the selectivity to the by-product isoprene was 0.064%. The measurement results are shown in Table 1.
Embodiment 3-7
[0044] Except for changing the reaction temperature, reaction pressure, reaction time, and the molar ratio of 2-methyl-3-buten-2-ol to 2-methoxypropene as shown in Table 1, the same as in Example 1 The method obtains colorless and transparent liquid products, which are respectively 165.63g, 164.24g, 167.59g, 162.00g, and 160.26g.
[0045] The obtained product was confirmed to be methylheptenone by gas mass spectrometry, and the same gas chromatography internal standard method as in Example 1 was used for measurement to obtain the conversion rate of the raw material 2-methyl-3-buten-2-ol and the product The selectivity of ylheptenone and by-product isoprene. The measurement results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com