Method for catalyzing conversion of carbonyl sulfide by using organic catalyst to synthesize five-membered sulfur heterocyclic compound
An organic catalyst and carbonyl sulfide technology, which is applied in the field of organocatalyst-catalyzed conversion of carbonyl sulfide to synthesize five-membered sulfur heterocyclic compounds, can solve the problems of poor applicability of reaction substrates and low reaction activity, and achieve simple and easy-to-obtain reaction raw materials and reagents, The post-processing process is simple and the operation is safe and simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
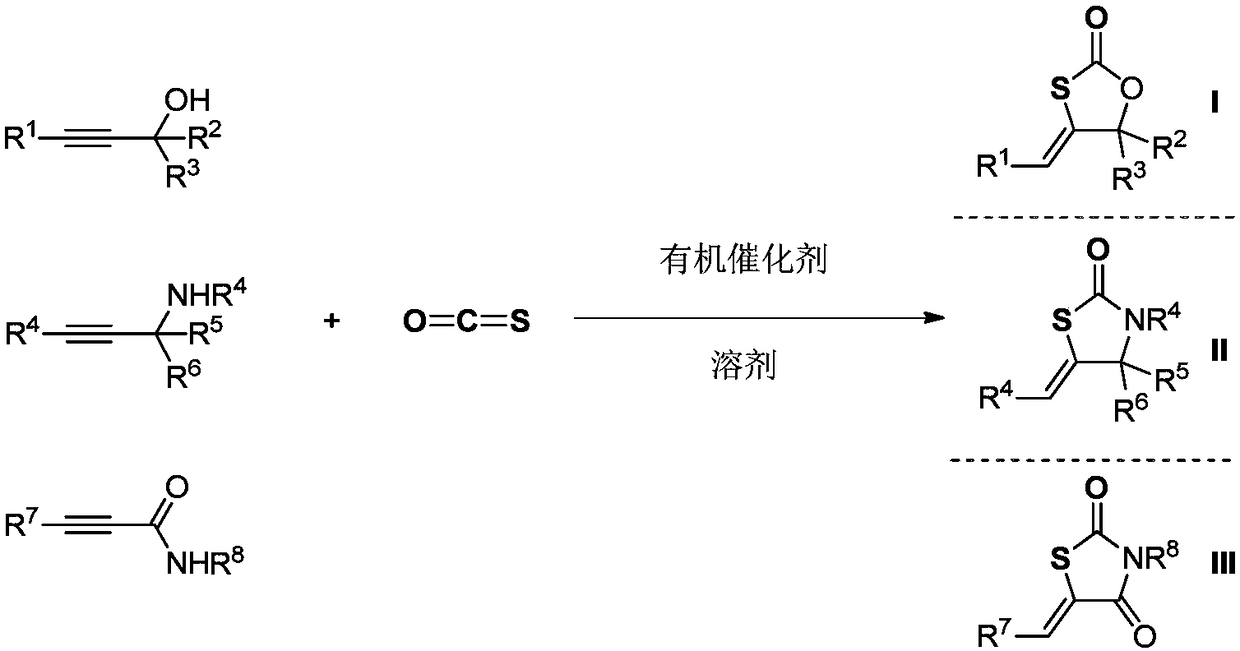
[0032] In a 20 milliliter autoclave, add a stirring bar, 5.0 millimoles of 2-methyl-4-benzene-3-butyn-2-alcohol, 0.025 millimoles of THPE-COS (n=1, R 13 benzyl), after stirring at 60 degrees Celsius for 1 hour, stop heating and stirring, cool to room temperature, and slowly release unreacted carbonyl sulfide. The reaction solution in the reaction kettle was dissolved in 2 ml of dichloromethane and transferred to a 50-ml round-bottom single-necked flask, and the reaction kettle was rinsed with (3×2 ml) of dichloromethane, and then the crude product was obtained after removing the solvent in vacuo . The crude product was separated and purified by column chromatography (developing solvent: dichloromethane). The yield was 95%.
Embodiment 2
[0034] In 20 milliliter autoclave, add stirring bar, 5.0 millimoles of 2-methyl-3-butyn-2-alcohol, 0.025 millimoles of THPE-COS (n=1, R 13 benzyl), after stirring at 60 degrees Celsius for 1 hour, stop heating and stirring, cool to room temperature, and slowly release unreacted carbonyl sulfide. The reaction solution in the reaction kettle was dissolved in 2 ml of dichloromethane and transferred to a 50-ml round-bottom single-necked flask, and the reaction kettle was rinsed with (3×2 ml) of dichloromethane, and then the crude product was obtained after removing the solvent in vacuo . The crude product was separated and purified by column chromatography (developing solvent: dichloromethane). The yield was 92%.
Embodiment 3
[0036] In a 20 milliliter autoclave, add a stirring bar, 5.0 millimoles of 2-phenyl-3-butyn-2-alcohol, 0.025 millimoles of THPE-COS (n=1, R 13 For methyl), after stirring at 50 degrees Celsius for 2 hours, stop heating and stirring, cool to room temperature, and slowly release unreacted carbonyl sulfide. The reaction solution in the reaction kettle was dissolved in 2 ml of dichloromethane and transferred to a 50-ml round-bottom single-necked flask, and the reaction kettle was rinsed with (3×2 ml) of dichloromethane, and then the crude product was obtained after removing the solvent in vacuo . The crude product was separated and purified by column chromatography (developing solvent: dichloromethane). The yield was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com