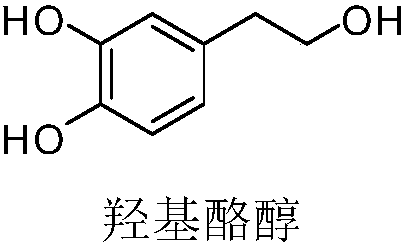
Improved method of hydroxytyrosol synthesizing process
A technology of hydroxytyrosol and dihydroxyphenylacetic acid, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as long production cycle, environmental pollution, and easy oxidation of products, and achieve less waste and more efficient operation Safe, low-cost results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] In order to better understand the content of the present invention, the technical solutions of the present invention will be further described below in conjunction with specific implementations, but the specific implementations do not limit the present invention.
[0021] Add 0.1 mol of a dichloromethane solution of tetrabutylammonium borohydride (preparation method reference J. Agric. Food Chem. 2000, 48, 4087-4090) into the reaction flask, and cool down to -5°C. In addition, 3,4-dihydroxyphenylacetic acid (8.4g, 0.05mol) was dissolved in 20ml of tetrahydrofuran, and added dropwise to the reaction flask, keeping the temperature at -5~5°C. After dropping, add iodomethane dropwise at this temperature ( 3.1ml, 0.05mol), the dropwise addition was completed, and the reaction was carried out at room temperature for 2 hours. Add about 80ml of 1N hydrochloric acid dropwise to the reaction solution, stir and separate the layers, extract the dichloromethane layer with water (40m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com