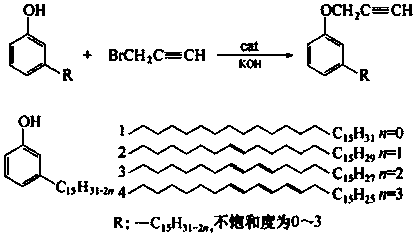
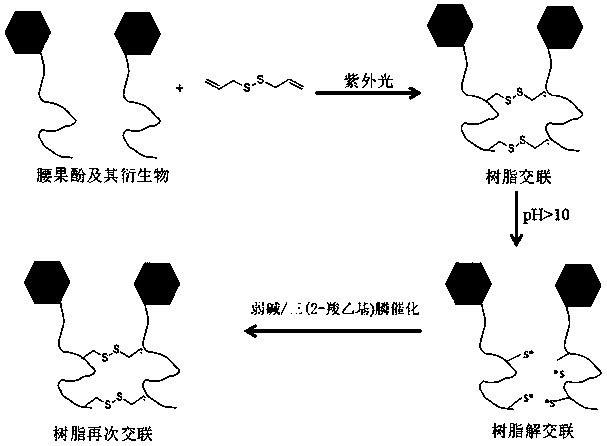
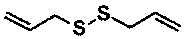Controllable and reversible cross-linked cardanol resin and preparation method thereof
A technology of cardanol resin and cardanol, which is applied in coatings and other directions, can solve the problems that cardanol is not easy to cross-link and solidify, cardanol resin is difficult to degrade and recycle, and cross-linking is uncontrollable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Cardanol and diallyl disulfide were dissolved in xylene accounting for 5w% of the total amount of the two at a mass ratio of 100:30 to obtain a controllable and reversible cross-linked cardanol resin. The controllable and reversible cross-linked cardanol resin was coated on the tinplate (thickness 100μm), under 350nm ultraviolet light (100μW / cm 2 ) under irradiation, the cardanol resin can be cross-linked and cured in 45s, and the cross-linking degree is 87.8%. The cross-linked and cured cardanol film was placed in the sodium ethoxide solution with pH=10 for 1 hour, and the cardanol film was decomposed and dissolved in the sodium ethoxide solution. The cardanol / sodium ethoxide solution was catalyzed by a weak base (pH=7.5) and tris(2-carboxyethyl)phosphine to reduce the sulfhydryl group to a disulfide bond, and the cardanol was re-crosslinked and solidified to precipitate, with a crosslinking degree of 68.3%.
[0021] Table 1 is the relationship between cardanol resin ...
Embodiment 2
[0024] Cardanol formal and diallyl disulfide were dissolved in xylene accounting for 5w% of the total amount of the two at a mass ratio of 100:25 to obtain a controllable and reversible cross-linked cardanol resin. Coat the controllable and reversible crosslinking resin on the tinplate (thickness 100μm), under 350nm ultraviolet light (100μW / cm 2 ) under irradiation, the cardanol formal resin can be cross-linked and cured in 60s, and the cross-linking degree is 83.4%. Put the cross-linked and cured paint film in the sodium ethoxide solution of pH=10 for 2 hours, the paint film decomposes and dissolves in the sodium ethoxide solution. The cardanol / sodium ethylate solution is catalyzed by a weak base (pH=7.5) and tris(2-carboxyethyl)phosphine to reduce the sulfhydryl group to a disulfide bond, and the cardanol formal is re-crosslinked, solidified and precipitated, with a crosslinking degree of 65.9 %.
[0025] Table 2 is the relationship between cardanol formal resin curing tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com