Methods and compositions for treating metabolic reprogramming disorders
A technology for reprogramming and disease, applied in drug combinations, active ingredients of phosphorus compounds, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0392] Treatment of immune disorders
[0393] Glutamine analogs of the present disclosure were found to inhibit the antigen-specific effector CD4 in vivo + T cell proliferation and function ( Figure 1C , Figure 1D and Figure 1E ) and antigen-specific CD8 + T cell response ( Figure 2A , 2B and Figure 2C ). Addition of DON to metabolic reprogramming therapy results in very potent inhibition of CD4 + T cell effector function and CD8 + T cell effector functions.
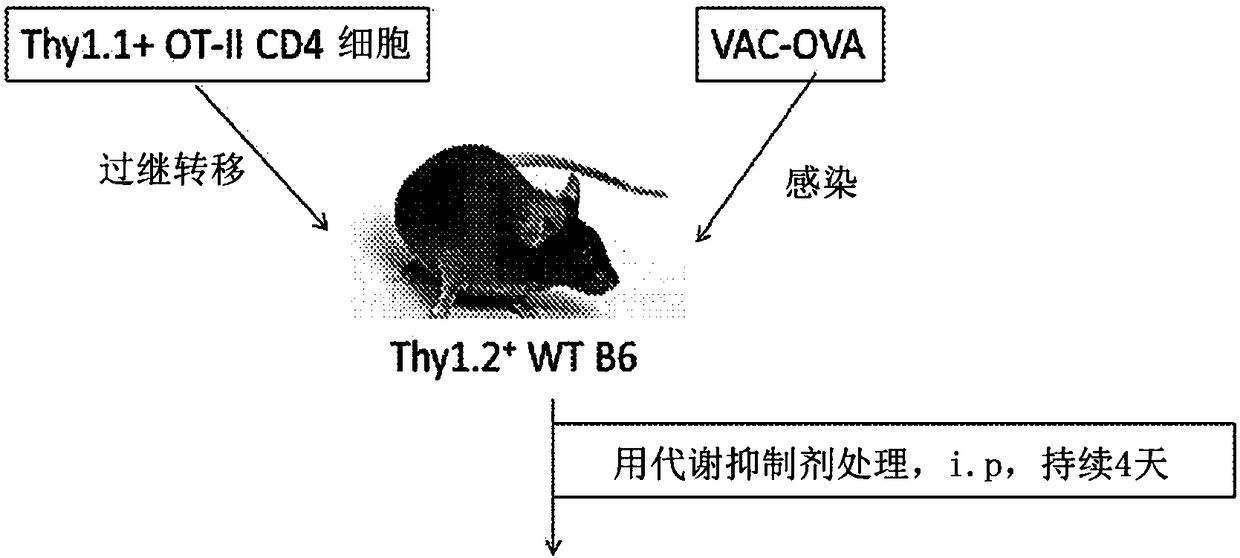
[0394] To determine whether this inhibition of T cells by glutamine analogues could be used to reduce transplant rejection, skin from Balb / c mice was transplanted into B6 mice and treated with at least three metabolic reprogramming agents (e.g., 2DG + metformin +DON) or treated with DON alone ( image 3 ). The results showed that DON prevented graft rejection and prolonged skin graft survival. Furthermore, when hearts from Balb / c mice were transplanted into B6 mice, DON was found to be able to prevent...
Embodiment 2
[0397] Modulation of immune metabolism as the sole means of preventing allograft rejection
[0398] Summary
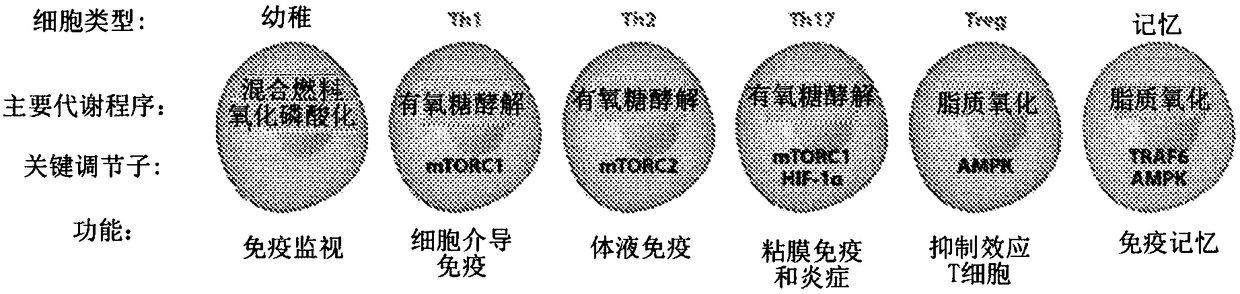
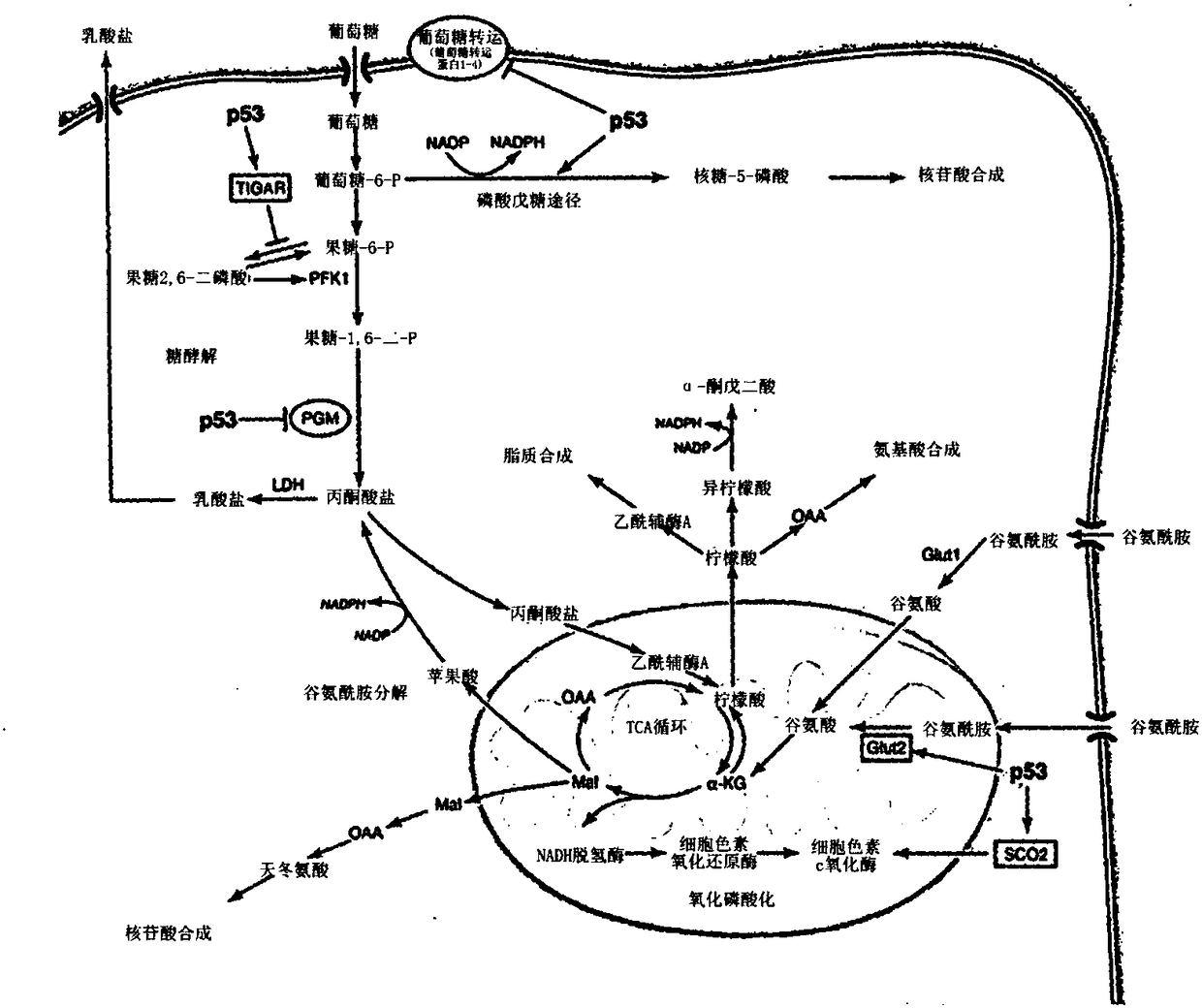
[0399] Upon antigen recognition and co-stimulation, T lymphocyte upregulation is a metabolic mechanism necessary for proliferation and maintenance of effector functions. Hallmarks of this response include a shift to aerobic glycolysis and an increased need for glutamine. This metabolic reprogramming in T cells is a key regulator of T cell activation and differentiation, not only as a result of antigen recognition. Furthermore, it is clear that while this metabolic reprogramming is important for the differentiation and function of T effector cells, the differentiation of regulatory T cells employs a different metabolic reprogramming. Based on these observations, it was hypothesized that inhibition of glycolysis and glutamine metabolism might represent a novel approach to prevent transplant rejection by inhibiting effector cell production and function while promoti...
Embodiment 3
[0433] Mice were sensitized to house dust mite antigen (HDM) in the absence of drugs. After intratracheal rechallenge, mice were treated with vehicle or 25. In this model, 25 suppressed pathology, recruitment / production of Th2 cells and reduced levels of HDM-specific IgE during acute lung rechallenge ( Figure 16 ). These data demonstrate the effectiveness of using DON prodrugs in the treatment of asthma.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com