Carbazole derivative and application thereof
An electroluminescent device and anode technology, which is applied in the direction of organic chemistry, luminescent materials, chemical instruments and methods, etc., can solve the problems of expensive precious metal raw materials, lack of main materials of deep blue light materials, etc., to reduce power consumption and improve fluorescence luminous efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
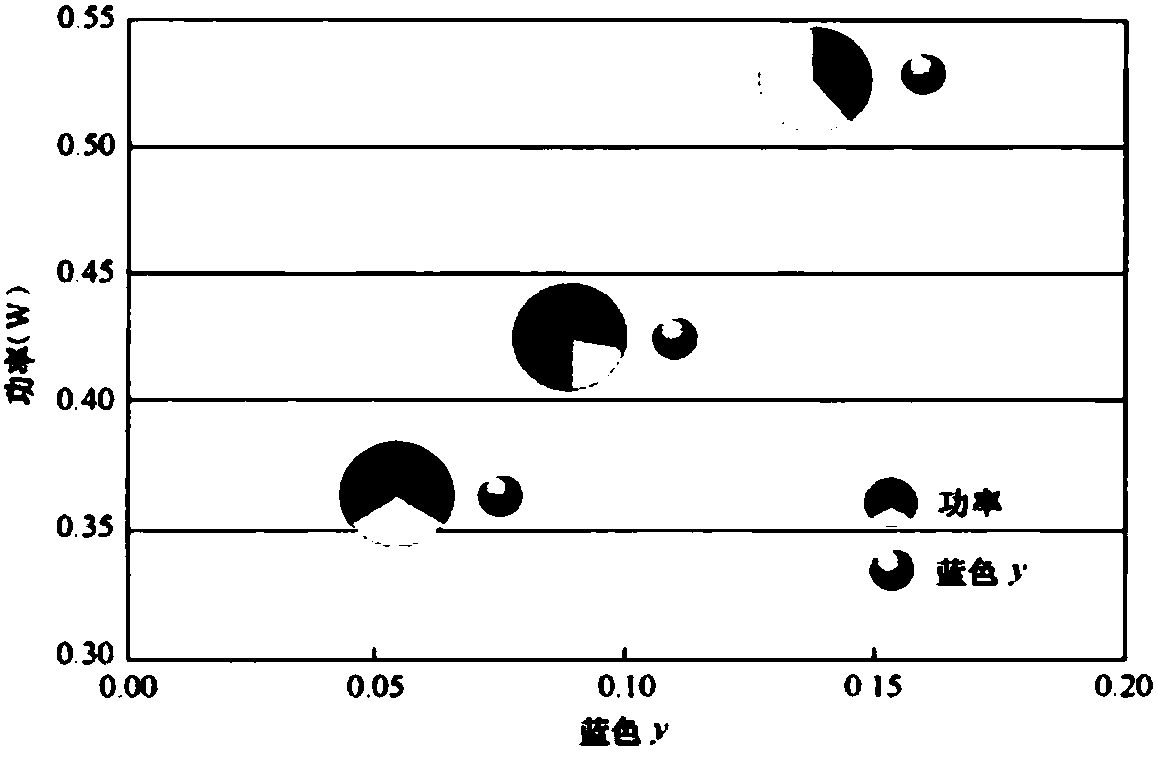
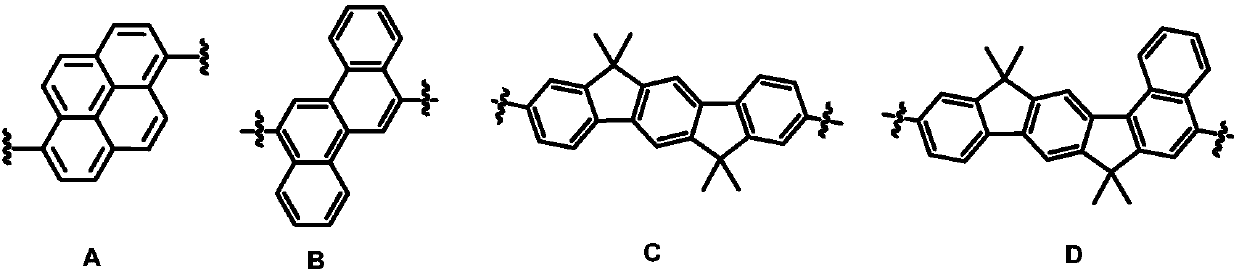
Image
Examples
Synthetic example 1
[0056]
[0057] Under argon flow, 4-bromo-9-phenylcarbazole (32.2g, 0.1mol), aniline (9.3g, 0.1mol), tris(dibenzylideneacetone) dipalladium ( 0) [Pd2(dba)3] 0.92g, tri-tert-butylphosphine 50% toluene solution 0.92ml, sodium tert-butoxide (19.2g, 0.2mol), dehydrated toluene 250mL, reflux reaction for 3 hours. After cooling, water and EA were added for extraction, the organic phase was filtered through celite and concentrated, and the obtained crude product was washed with ethanol to obtain a light yellow solid M1=21.9 g, yield 84.5%.
[0058] Under argon flow, put 1,6-dibromopyrene (3.6g, 0.01mol), N-[4-(N-phenylcarbazole)]-aniline (6.7g, 0.02mol) into a 250mL eggplant-shaped flask , Tris (dibenzylidene acetone) dipalladium (0) [Pd2 (dba) 3] 0.08g, tri-tert-butylphosphine 50% toluene solution 0.5ml, sodium tert-butoxide (2.9g, 0.03mol), dehydrated toluene 100mL, reflux reaction for 3 hours. After cooling, the reaction solution was filtered with diatomaceous earth, and the ...
Synthetic example 2
[0061] Compound A2 was prepared in the same manner as in Example 1, except that N-[4-(N-phenylcarbazole)]-aniline was replaced by equivalent N-[4-(N-phenylcarbazole)] -2-Methylaniline, after the reaction was completed, 6.7 g of white solid was isolated, with a yield of 75%.
Synthetic example 3
[0063] Compound A3 was prepared in the same manner as in Example 1, except that N-[4-(N-phenylcarbazole)]-aniline was replaced by equivalent N-[4-(N-phenylcarbazole)]- After the reaction was completed, 3-methylaniline was isolated to obtain 6.7 g of white solid, with a yield of 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com