A kind of promethazine hydrochloride tablet and preparation method thereof
A technology of promethazine hydrochloride tablets and promethazine hydrochloride, which is applied in medical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the pain, difficulty of carrying, and compliance of injections To achieve the effect of increasing compliance, improving taste, and simplifying prescriptions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
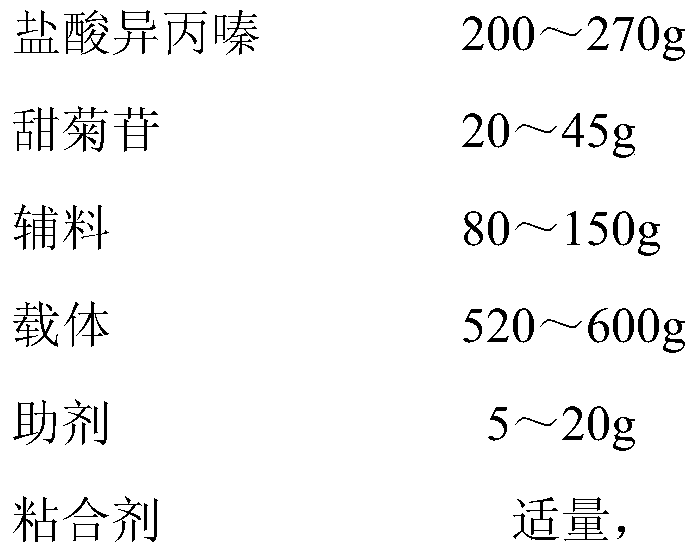
[0022] (1) Add 150mL ethanol to 150mL deionized water to make a uniform solution, and divide the resulting solution into three parts on average;
[0023] (2) Add 60g of Poloxamer 407 and 40g of PEG4000 to one of the solutions prepared in step (1), heat to 60°C and stir thoroughly to obtain a dispersion of excipients;
[0024] (3) 260g promethazine hydrochloride is added in another wherein a solution prepared in step (1), fully stirred to obtain the main material dispersion;
[0025] (4) fully mixing the auxiliary material dispersion obtained in step (2) and the main material dispersion obtained in step (3), and vacuum drying until the moisture content of the mixture is less than 1% (mass percentage);
[0026] (5) Grind the dried material in step (4) to 100-120 meshes, and mix it with 35g stevioside, 260g starch, and 260g microcrystalline cellulose in a granulator, and add it to step (1) For the remaining part of the solution prepared, set the frequency of the shearing knife t...
Embodiment 2
[0028] (1) Add 180mL ethanol to 120mL deionized water to make a uniform solution, and divide the obtained solution into three parts on average;
[0029] (2) Add 100g of PEG6000 to one of the solutions prepared in step (1), heat to 60°C and stir thoroughly to obtain a dispersion of excipients;
[0030] (3) 240g promethazine hydrochloride is added in another one of the solutions prepared in step (1), fully stirred to obtain the main material dispersion;
[0031] (4) fully mixing the auxiliary material dispersion obtained in step (2) and the main material dispersion obtained in step (3), and vacuum drying until the moisture content of the mixture is less than 1% (mass percentage);
[0032] (5) Grind the dried material in step (4) to 100-120 meshes, put it into a granulator with 30g of stevioside, 200g of starch, and 300g of microcrystalline cellulose and mix well, and add it to step (1) For the remaining part of the solution prepared, set the frequency of the shearing knife to 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com