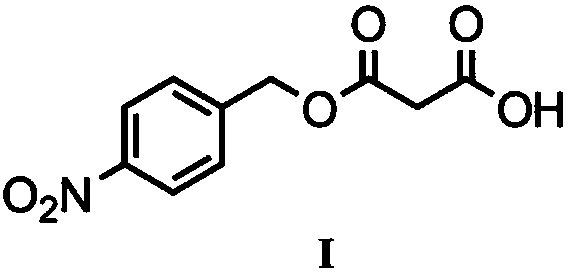
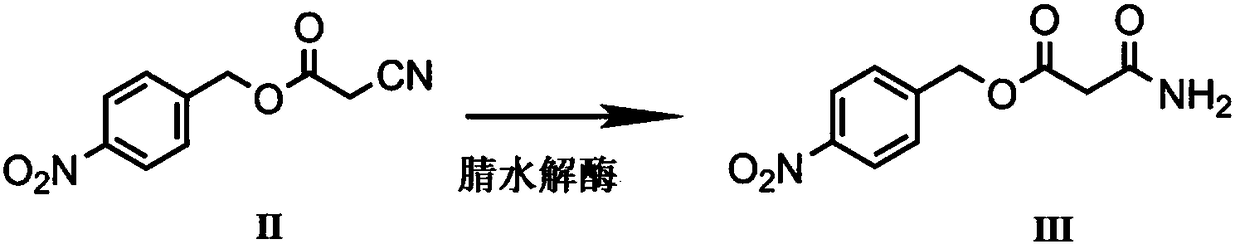
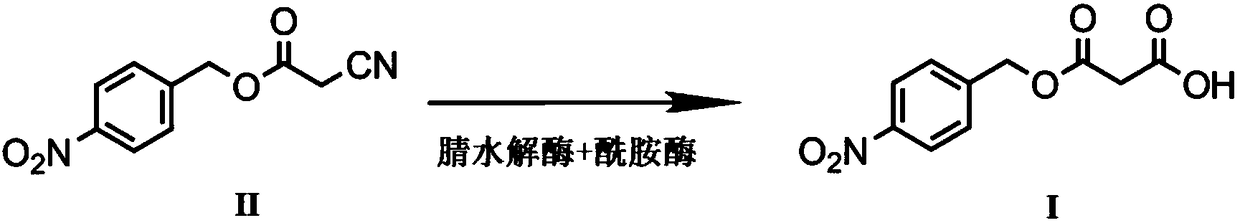
Biological preparation method of p-nitrobenzyl alcohol propandioic acid monoester
A technology of nitrobenzyl alcohol malonate monoester and microorganisms, applied in the field of biocatalysis, can solve the problems of uneconomical and low yield, reduce the production of amide by-product III, increase yield, and realize industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The construction of embodiment 1 nitrilase SEQ ID NO:1 expression strain
[0053] The gene sequence encoding nitrilase SEQ ID NO:1 was synthesized from the whole gene, and connected with the vector plasmid pET21a (purchased from Novagen) through Nde I and Xho I double restriction sites to obtain a recombinant plasmid. The Escherichia coli DH5α competent was transformed by calcium chloride method, and the genetic engineering plasmid with correct DNA sequence and capable of expressing nitrilase was obtained after culturing and extracting the plasmid. The genetic engineering plasmid constructed above was transformed into competent Escherichia coli BL21(DE3) by the calcium chloride method, coated on an LA plate, placed in a constant temperature incubator at 37°C, and cultured upside down overnight. On the next day, pick 3-5 positive clones from the LA plate, and perform IPTG-induced enzyme production test on the positive clones. For the positive identification above, it is...
Embodiment 2
[0054] The construction of embodiment 2 amidase SEQ ID NO:2 expression strain
[0055] The gene sequence encoding amidase SEQ ID NO: 2 was synthesized from the whole gene, and connected with the vector plasmid pET21a (purchased from Novagen) through Nde I and Xho I double restriction sites to obtain a recombinant plasmid. The Escherichia coli DH5α competent was transformed by calcium chloride method, and the genetic engineering plasmid with correct DNA sequence and amidase expression was obtained after culturing and extracting the plasmid. The genetic engineering plasmid constructed above was transformed into competent Escherichia coli BL21(DE3) by the calcium chloride method, coated on an LA plate, placed in a constant temperature incubator at 37°C, and cultured upside down overnight. On the next day, pick 3-5 positive clones from the LA plate, and perform IPTG-induced enzyme production test on the positive clones. For the positive identification above, it is the engineering...
Embodiment 3
[0056] Embodiment 3 Escherichia coli engineering strain shake flask fermentation
[0057] 3.1 The nitrilase-producing Escherichia coli engineered strain obtained in Example 1 was inoculated into LB medium, and placed on a constant temperature shaker at 37° C. at 200 rpm for shaking flask culture overnight. The next day, add IPTG to a final concentration of 0.5mmol / L, and induce at 30°C and 200rpm for 6-8 hours. Subsequently, the cells containing nitrilase were collected by centrifugation at 4° C. (6000 rpm, 5 min). Wash the cells containing nitrilase twice with 0.9% sodium chloride solution. Discard the sodium chloride solution for the last wash, and store the cells containing nitrilase in a -80°C refrigerator.
[0058] 3.2 Inoculate the amidase-producing Escherichia coli engineered strain obtained in Example 2 into LB medium, and place it on a constant temperature shaker at 37° C. at 200 rpm for shaking flask culture overnight. The next day, add IPTG to a final concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com