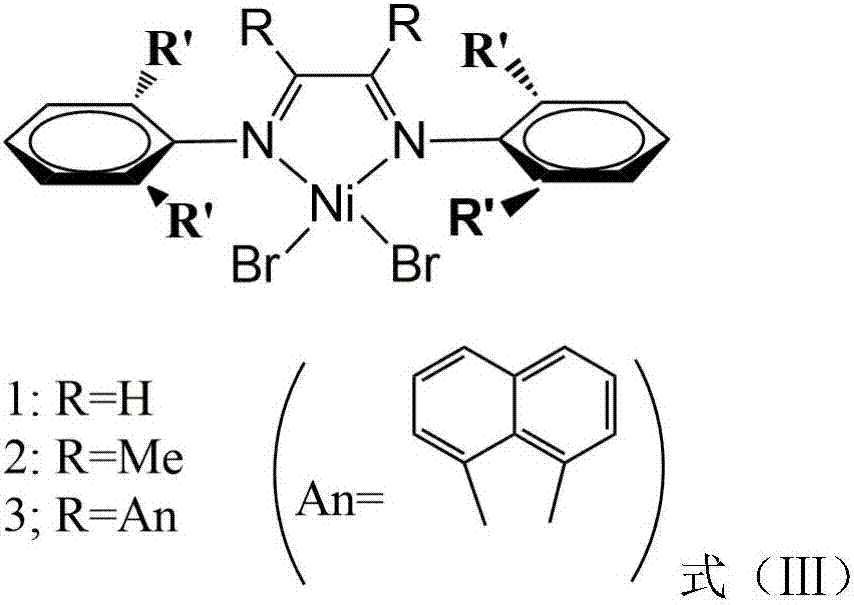
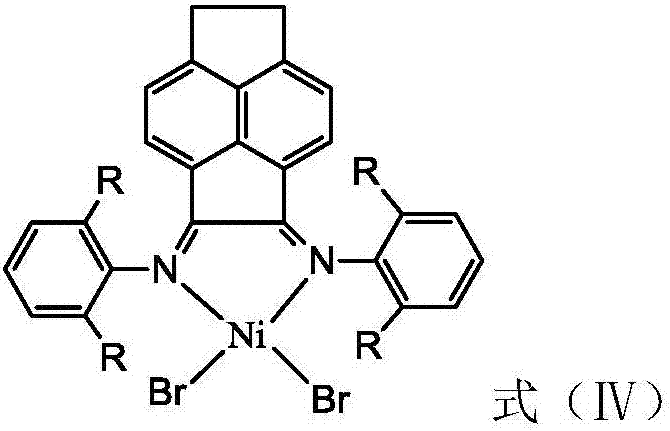
Vinylene acenaphthene (alpha-diimine) nickel olefin catalyst, and preparation method and application thereof
An olefin catalyst and vinylidene technology, applied in chemical instruments and methods, nickel organic compounds, organic chemistry, etc., can solve the problems of inability to prepare high-molecular-weight, high-branched polyethylene, etc., and achieve reduced production costs and high activity , good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of described vinylidene acenaphthylene (α-diimine) nickel olefin catalyst comprises the following steps:
[0052] 1) Compound C1 is obtained by diacylation of acenaphthene: using acenaphthene as a raw material and carbon disulfide as a solvent. Anhydrous aluminum bromide was added as a catalyst to obtain yellow solid acenaphthylenedione C1 under the condition of oxalyl bromide as an oxidizing agent.
[0053]
[0054] 2) compound C1 is subjected to bromination reaction to obtain compound C2: taking compound C1 obtained in step (1) as raw material, carbon tetrachloride as solvent, benzoyl peroxide as initiator, N-bromosuccinimide (NBS ) as brominating agent, compound C2 can be obtained by bromination reaction.
[0055]
[0056] 3) Compound C2 undergoes an elimination reaction to obtain compound C3: using compound C2 obtained in step (2) as a raw material, acetone as a solvent, and anhydrous potassium iodide as an initiator, acenaphthylenedion...
Embodiment 1
[0069] 2g (9.6mmol) C1 is added in the 500mL there-necked bottle that 250mL carbon tetrachloride is housed, N 2 Reflux at 85°C for 30min under protection, add 5.2g (30mmol) N-bromosuccinimide (NBS) and 200 mg benzoyl peroxide to the system, and continue to reflux the mixture at 85°C for 5h. After the reaction is over, filter while hot, and use 50mL hot CCl 4 After washing, the mixture obtained by washing was subjected to rotary evaporation, and the solvent carbon tetrachloride was removed to obtain a yellow solid, which was a mixture of product C2 and NBS. Purified by column chromatography with dichloromethane as the eluent, the total mass of product C2 was 2.311 g, and the yield was 65.57%.
[0070] 1 H-NMR (400MHz, CDCl 3 , δin ppm): 8.25 (dd, 2H, Ar-H), 7.91 (dd, 2H, Ar-H), 6.09 (s, 2H, CH).
[0071] Two, the preparation of compound C3
Embodiment 2
[0073] Dissolve 1.25g (34.2mmol) C2 in 100mL acetone under the protection of nitrogen, add the acetone solution into a 250mL three-necked flask, then add 7.5g (0.46mol) anhydrous potassium iodide to the three-necked flask, and at 60°C Reflux 4h. After the reaction was over, the mixture was cooled and poured into an aqueous solution of sodium thiosulfate, the mixture was extracted three times with chloroform, then washed three times with deionized water, and the red product C3 was obtained after rotary evaporation, with a yield of 0.960 g and a yield of 99.92% .
[0074] 1 H-NMR (400MHz, CDCl 3 , δin ppm): 8.26 (dd, 2H, Ar-H), 8.13 (dd, 2H, Ar-H), 7.69 (s, 2H, CH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com