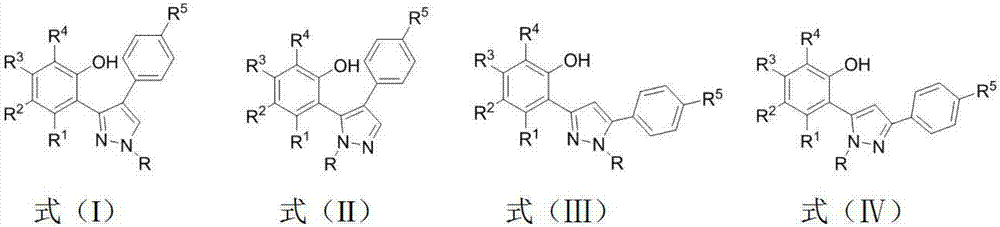
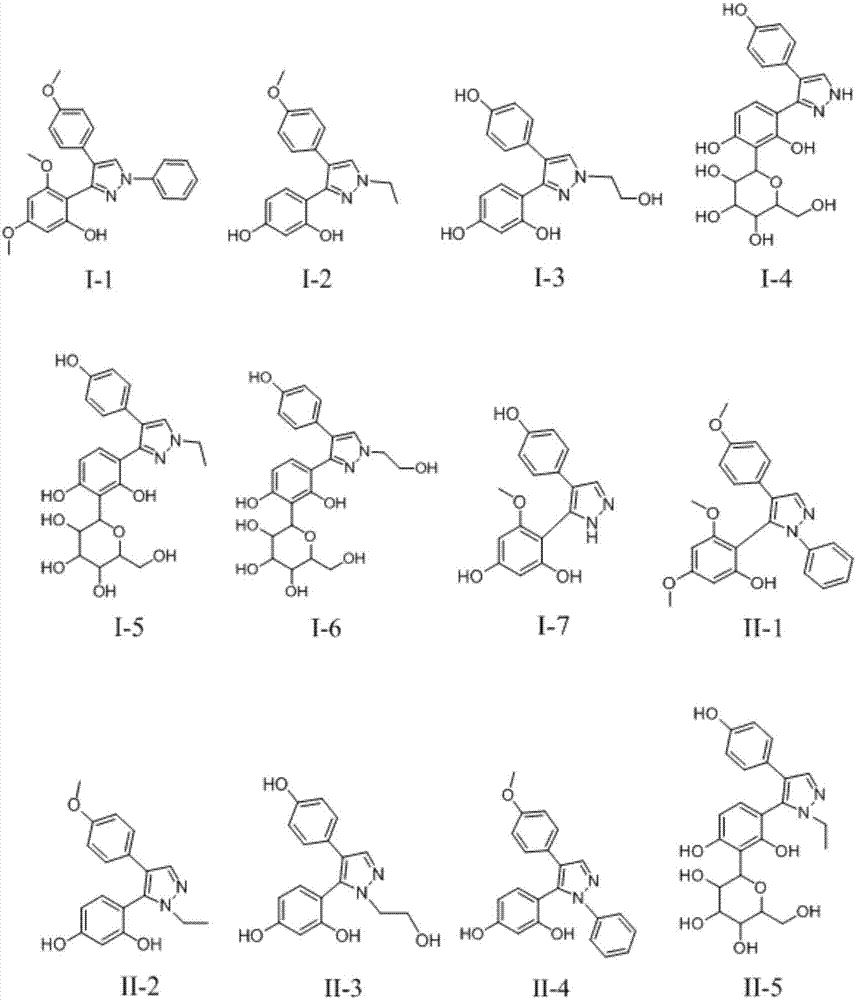
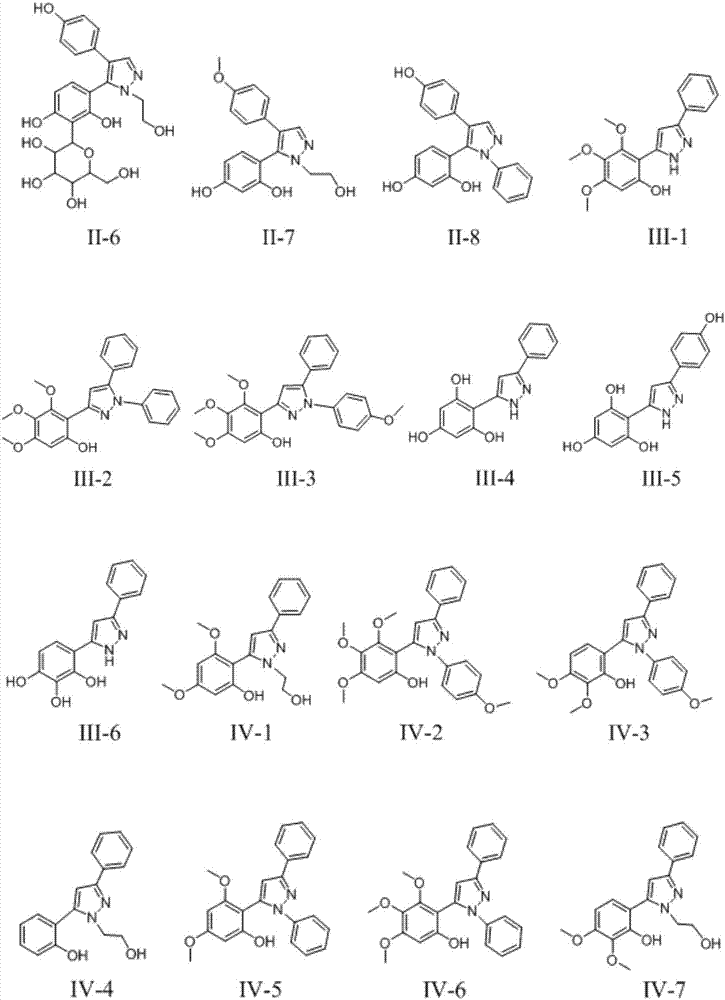
3,5-diaryl pyrazole or 3,4-diaryl pyrazole derivative and application thereof
A technology of diarylpyrazoles and diarylpyrazoles is applied in the field of 3,5-diarylpyrazole and 3,4-diarylpyrazole derivatives and their preparation, and can solve the problem of drug resistance Serious problems such as good reversal of antibacterial drug resistance, good antifungal infection activity, and good economic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of compound I-1 and II-1
[0031]
[0032] Get genistein (I) 400mg and place round bottom flask, then add 1.8g K 2 CO 3 , add 50 mL of anhydrous acetone to dissolve, then add 800 μL of iodomethane, and reflux at 60°C overnight. After the reaction was completed, acetone was removed by rotary evaporation, water-ethyl acetate was added for extraction, and the organic phase was evaporated to dryness under pressure to obtain compound II as a yellow solid (yield 95%).
[0033] Take 100 mg of compound II in a round bottom flask, add 10 mL of ethanol to dissolve, then add 300 μL of phenylhydrazine, and heat at 90°C for 48 hours. After the reaction was completed, the solvent was removed under reduced pressure and passed through a silica gel column to obtain yellow compounds I-1 (22 mg, yield 22%) and II-1 (77 mg, yield 77%).
[0034] Ⅰ-1: MS m / z 403 (M+1); 1H NMR (400MHz, CDCl3) δ = 7.99 (s, 1H), 7.26 (m, 7H), 6.82 (d, J = 8.8, 2H), 6.12 (d,J...
Embodiment 2
[0036] Embodiment 2: the preparation of compound III-4
[0037]
[0038] Take 400mg of 7,8-dihydroxyflavone (Ⅲ) and place it in a round bottom flask, then add 1.5g K 2 CO 3 , add 50mL of anhydrous acetone to dissolve, then add 500μL of iodomethane, and reflux at 60°C overnight. After the reaction was completed, acetone was removed by rotary evaporation, water-ethyl acetate was added for extraction, and the organic phase was evaporated to dryness under pressure to obtain compound IV as a yellow solid (yield 90%).
[0039] Take 100 mg of compound IV in a round bottom flask, add 10 mL of ethanol to dissolve, then add 100 μL of hydrazine hydrate, and heat at 100 ° C for 24 h. After the reaction was completed, the solvent was removed under reduced pressure, and extracted with water-ethyl acetate to obtain 92 mg of tan solid V (92% yield).
[0040] Take 50 mg of compound V in a round bottom flask, dissolve it in 6 mL of anhydrous DCM, cool down to -78°C, add 2 mL of 1M boron tri...
Embodiment 3
[0042] Embodiment 3: the preparation of compound I-2 and II-2
[0043]
[0044] Take 100mg of formononetin VI, dissolve it in 6mL of absolute ethanol, add 200mg of ethylhydrazine maleic acid, stir at 100°C for 12h to complete the reaction, evaporate the solvent to dryness under reduced pressure, and pass through a silica gel column. 35 mg of I-2 (yield 35%) and 50 mg of II-2 (yield 50%) were obtained.
[0045] Ⅰ-2: MS m / z 311 (M+1); 1H NMR (400MHz, MeOD) δ = 7.60 (d, J = 2.7, 1H), 7.19 (d, J = 7.8, 2H), 6.97 (d, J=8.5,1H),6.86(d,J=7.9,2H),6.36(d,J=1.6,1H),6.12(dd,J=8.5,2.4,1H),4.17(dd,J=7.3, 1.6, 2H), 3.77 (d, J = 0.9, 3H), 1.55–1.41 (m, 3H).
[0046] Ⅱ-5: MS m / z 310 (M+1); 1H NMR (400MHz, MeOD) δ = 7.70 (s, 1H), 7.15 (d, J = 8.8, 2H), 6.80 (d, J = 8.3, 1H), 6.76(d, J=8.8, 2H), 6.45(d, J=2.2, 1H), 6.35(dd, J=8.3, 2.3, 1H), 3.96(q, J=7.2, 2H), 3.80 -3.56 (m, 3H), 1.30 (t, J = 7.2, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com