A kind of esomeprazole sodium freeze-dried powder and preparation method thereof
A technology of esomeprazole sodium and omeprazole sodium, which is applied in the field of esomeprazole sodium freeze-dried powder and its preparation, can solve potential safety hazards, large errors, unrealistic production operations, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
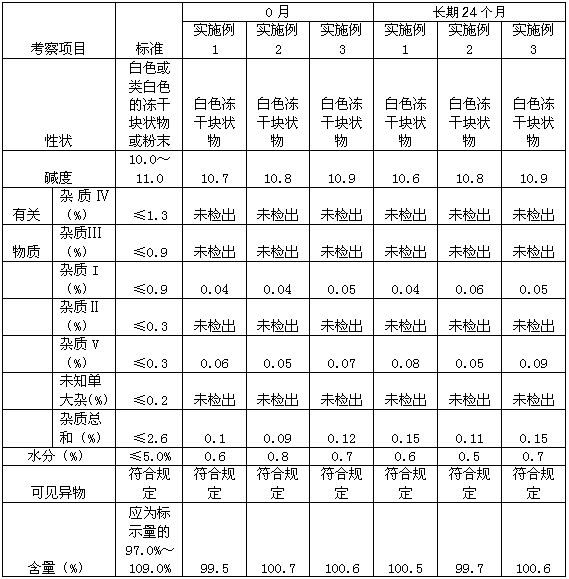
Examples
Embodiment 1
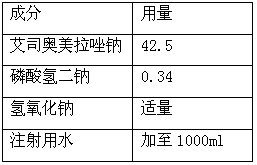
[0027] A freeze-dried powder of esomeprazole sodium (1000 freeze-dried powder injections), the composition is as follows:
[0028]
[0029] Follow the steps below to prepare:
[0030] 1. Prepare the solution
[0031] (1) Cool the water for injection to 10°C for later use;
[0032] (2) Add 80% of the prepared volume of water for injection into the dispensing tank, add the prescribed amount of disodium hydrogen phosphate and esomeprazole sodium, stir to dissolve completely;
[0033] (3) Use 0.1mol / L sodium hydroxide solution to adjust the pH value of the liquid to 10.5;
[0034] (4) Add 0.05% (g / mL) activated carbon and stir for 15 minutes;
[0035] (5) First decarbonize by filtering through a 0.8μm filter membrane, add water for injection to the full amount, then filter through a 0.22μm filter membrane, fill and fill with a half stopper.
[0036] 2. Freeze-drying
[0037] Put the above-mentioned subpackaged semi-finished products into the freeze-drying box;
[0038] ...
Embodiment 2
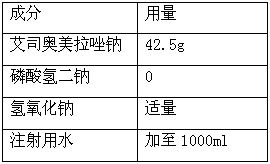
[0042] A freeze-dried powder of esomeprazole sodium (1000 freeze-dried powder injections), the composition is as follows:
[0043]
[0044] Follow the steps below to prepare:
[0045] 1. Prepare the solution
[0046] (1) Cool the water for injection to 12°C for later use;
[0047] (2) Add 80% of the prepared volume of water for injection into the dispensing tank, add the prescribed amount of disodium hydrogen phosphate and esomeprazole sodium, stir to dissolve completely;
[0048] (3) Use 0.1mol / L sodium hydroxide solution to adjust the pH value of the liquid to 10.8;
[0049](4) Add 0.08% (g / mL) activated carbon and stir for 15 minutes;
[0050] (5) First decarbonize by filtering through a 0.8μm filter membrane, add water for injection to the full amount, then filter through a 0.22μm filter membrane, fill and fill with a half stopper.
[0051] 2. Freeze-drying
[0052] Put the above-mentioned subpackaged semi-finished products into the freeze-drying box;
[0053] ...
Embodiment 3
[0057] A freeze-dried powder of esomeprazole sodium (1000 freeze-dried powder injections), the composition is as follows:
[0058]
[0059] Follow the steps below to prepare:
[0060] 1. Prepare the solution
[0061] (1) Cool the water for injection to 15°C for later use;
[0062] (2) Add 80% of the prepared volume of water for injection into the dispensing tank, add the prescribed amount of disodium hydrogen phosphate and esomeprazole sodium, stir to dissolve completely;
[0063] (3) Use 0.1mol / L sodium hydroxide solution to adjust the pH value of the liquid to 11.0;
[0064] (4) Add 0.1% (g / mL) activated carbon and stir for 15 minutes;
[0065] (5) First decarbonize by filtering through a 0.8μm filter membrane, add water for injection to the full amount, then filter through a 0.22μm filter membrane, fill and fill with a half stopper.
[0066] 2. Freeze-drying
[0067] Put the above-mentioned subpackaged semi-finished products into the freeze-drying box;
[0068] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com