Preparation method for perfluoro-substituted disulfonic anhydride
A technology of disulfonic anhydride and ethylene disulfonic anhydride, which is applied in the field of preparation of perfluoro-substituted disulfonic anhydride and perfluoro-substituted disulfonic anhydride. Problems, achieve the effect of improving charge and discharge performance and cycle times, small equipment investment, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
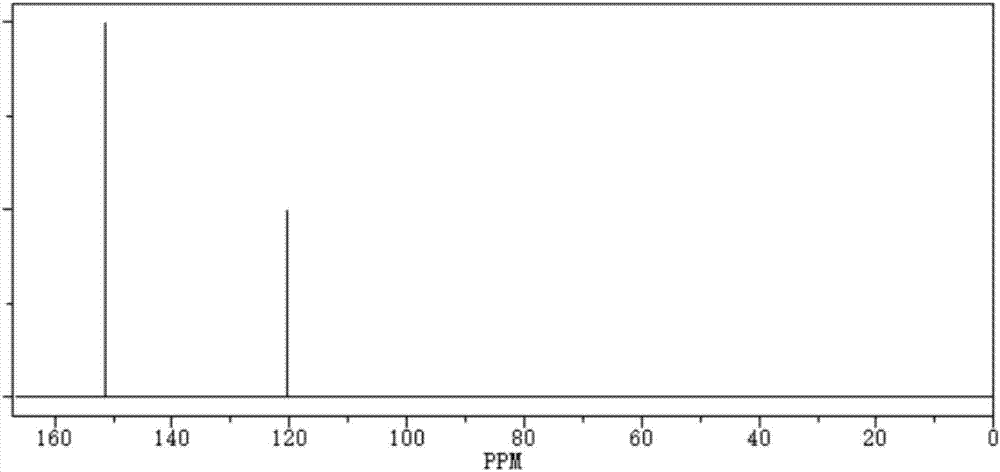
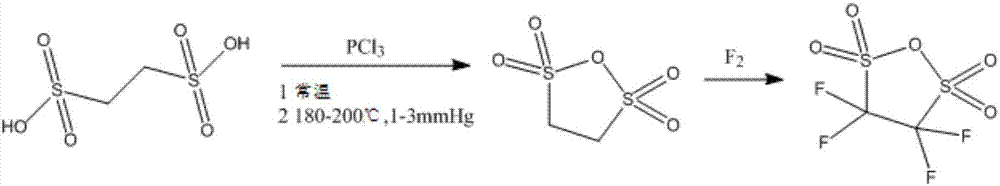
[0025] Take 0.1mol (19g) of ethanedisulfonic acid, add molecular sieves, add 76g of phosphorus trichloride and react at room temperature for 5 hours, then raise the temperature to 180°C, continue to react for 3 hours under a vacuum of 1-3mmHg, and then extract with dichloromethane, Crystallization was carried out to obtain 17.05g of ethanedisulfonic anhydride with a yield of 99.13%;
[0026] Fluorine gas was passed into the ethanedisulfonic anhydride obtained above, and the molar ratio of ethanedisulfonic anhydride to fluorine gas was controlled to be 1:6 to prepare 23.5 g of perfluoro-substituted ethanedisulfonic anhydride with a yield of 97.19%. The total yield was calculated to be 96.34%.
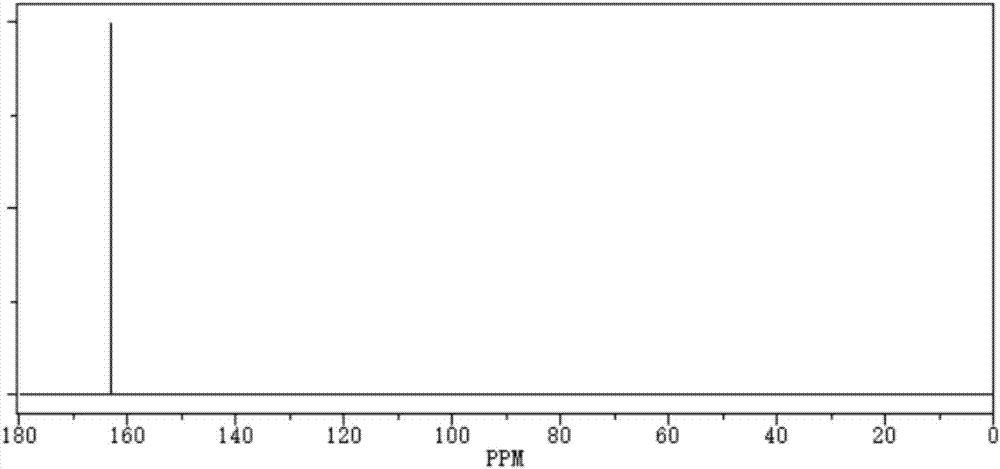
[0027] The purity of the obtained perfluoro-substituted ethanedisulfonic anhydride is 99.8%, the boiling point is 259.3°C, 760mmHg, and the density is 2.18g / cm 3 , The impurity content of ethanedisulfonic acid and difluoro-substituted disulfonic anhydride is less than 1ppm.
Embodiment 2
[0029] Take 0.1mol (19g) of ethanedisulfonic acid, add molecular sieves, add 57g of phosphorus trichloride and react at room temperature for 8 hours, then raise the temperature to 200°C, continue to react for 5 hours under a vacuum of 1-3mmHg, and then extract with dichloromethane, Crystallization, to obtain 16.75g of ethanedisulfonic anhydride, the yield is 97.38%;
[0030] Fluorine gas was passed into the ethanedisulfonic anhydride obtained above, and the molar ratio of ethanedisulfonic anhydride to fluorine gas was controlled to be 1:4, and 22.9 g of perfluoro-substituted ethanedisulfonic anhydride were prepared with a yield of 96.4%. The calculated total yield was 93.87%.
[0031] The purity of the obtained perfluoro-substituted ethanedisulfonic anhydride is 99.85%, the boiling point is 259.6°C, 760mmHg, and the density is 2.17g / cm 3 , The impurity content of ethanedisulfonic acid and difluoro-substituted disulfonic anhydride is less than 1ppm.
Embodiment 3
[0033] Take 0.1mol (19g) of ethanedisulfonic acid, add molecular sieves, add 95g of phosphorus trichloride and react at room temperature for 6 hours, then raise the temperature to 190°C, continue to react for 2 hours under a vacuum of 1-3mmHg, and then extract with dichloromethane, Crystallization, to obtain 16.89g ethanedisulfonic anhydride, the yield is 98.2%;
[0034] Fluorine gas was passed into the ethanedisulfonic anhydride obtained above, and the molar ratio of ethanedisulfonic anhydride to fluorine gas was controlled to be 1:7, and 23.14 g of perfluoro-substituted ethanedisulfonic anhydride was prepared with a yield of 96.58%. The calculated total yield was 94.84%.
[0035] The purity of the obtained perfluoro-substituted ethanedisulfonic anhydride is 99.87%, the boiling point is 259.2°C, 760mmHg, and the density is 2.19g / cm 3 , The impurity content of ethanedisulfonic acid and difluoro-substituted disulfonic anhydride is less than 1ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com