Chemically modified fusidic acid, preparation method thereof and application of chemically modified fusidic acid
The technology of geoacid chemistry and fusidic acid is applied in the field of chemical modification of fusidic acid and its preparation, which can solve the problems of antibiotic drug abuse, influence human health and safety, etc., and achieves high synthesis yield, simple preparation method and application. wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
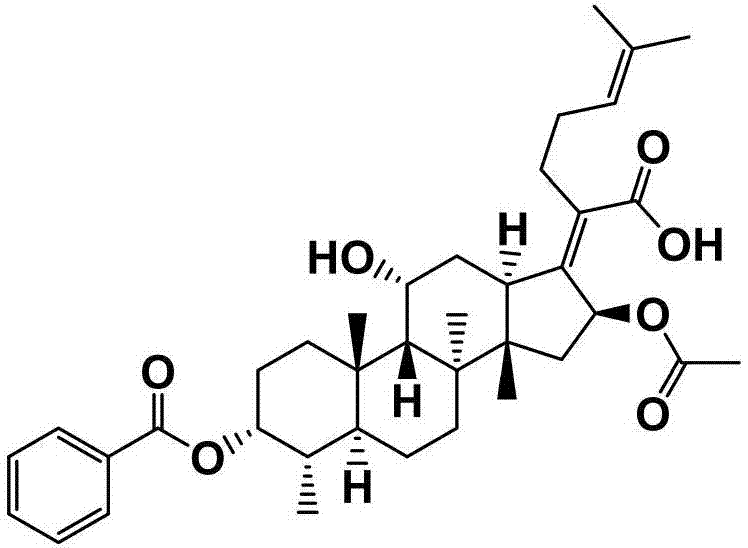
[0055] The preparation of embodiment 1 fusidic acid chemical modification (FA-E-01)
[0056] First weigh 300mg (0.581mmol) of fusidic acid and 142mg (1.161mmol) of 4-dimethylaminopyridine (DMAP) in a 50mL round-bottomed flask containing magnets, and measure 10mL under the condition of nitrogen protection Put ultra-dry anhydrous dichloromethane in a flask, and stir for about 15 minutes at room temperature and under nitrogen. After it is completely dissolved, add 0.94 mL (1.161 mmol) of pyridine with a syringe, and stir for about After 20 min, 0.20 mL (1.743 mmol) of three times the amount of benzoyl chloride was finally added, and the reaction was stirred for 2 h at room temperature under nitrogen protection. The end point of the reaction was detected by TLC (developing agent: dichloromethane: ethyl acetate = 3: 1, chromogen: methanol: acetic acid: concentrated sulfuric acid: anisaldehyde (volume ratio) = 85: 10: 5: 0.5), the reaction was completed , the crude product of compo...
Embodiment 2
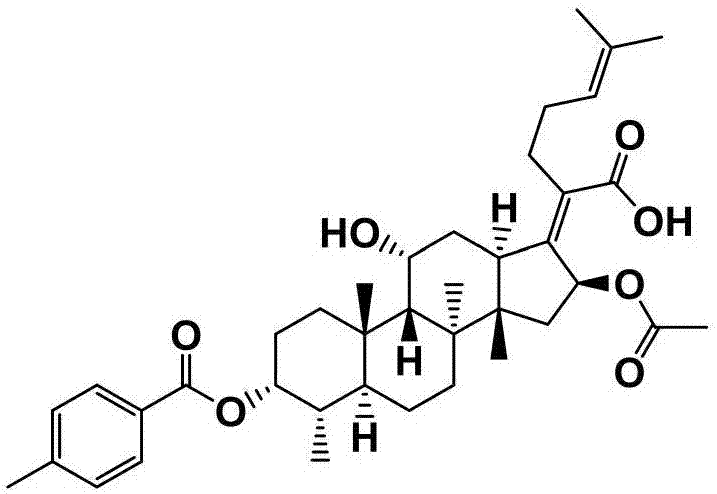
[0057] Preparation of Example 2 Fusidic Acid Chemical Modifications (FA-E-02)
[0058] First weigh 300mg (0.581mmol) of fusidic acid and 142mg (1.161mmol) of 4-dimethylaminopyridine (DMAP) in a 50mL round-bottomed flask containing magnets, and measure 10mL under the condition of nitrogen protection Put ultra-dry anhydrous dichloromethane in a flask, stir for about 15 min at room temperature and nitrogen protection, and then add 0.94 mL (1.161 mmol) of pyridine with a syringe, and stir at room temperature and nitrogen After about 20 minutes, three times the amount of p-toluoyl chloride 0.23 mL (1.743 mmol) was finally added, and the reaction was stirred for 2 hours at room temperature under nitrogen protection. The end point of the reaction was detected by TLC (developing agent: dichloromethane: ethyl acetate = 3: 1, chromogen: methanol: acetic acid: concentrated sulfuric acid: anisaldehyde (volume ratio) = 85: 10: 5: 0.5), the reaction was completed , the crude product of com...
Embodiment 3
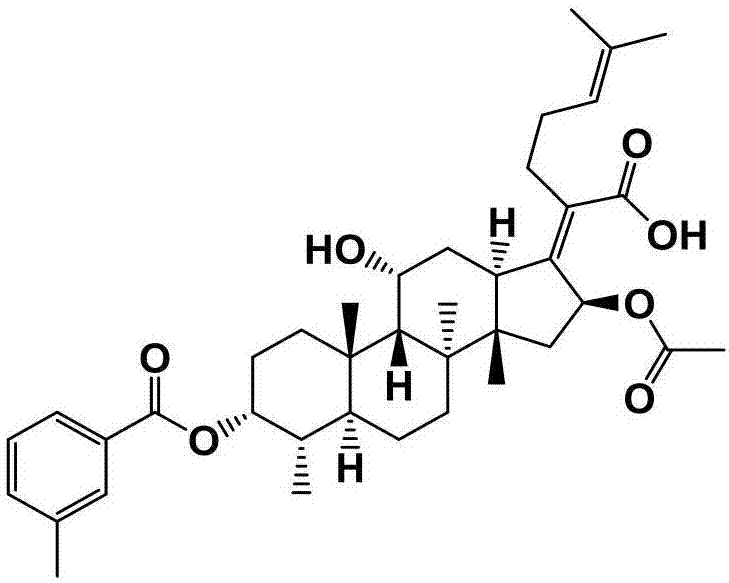
[0059] Preparation of Example 3 Fusidic Acid Chemical Modifications (FA-E-03)
[0060] First weigh 300mg (0.581mmol) of fusidic acid and 142mg (1.161mmol) of 4-dimethylaminopyridine (DMAP) in a 50mL round-bottomed flask containing magnets, and measure 10mL under the condition of nitrogen protection Put ultra-dry anhydrous dichloromethane in a flask, stir for about 15 min at room temperature and nitrogen protection, and then add 0.94 mL (1.161 mmol) of pyridine with a syringe, and stir at room temperature and nitrogen After about 20 minutes, three times the amount of m-toluoyl chloride 0.23 mL (1.743 mmol) was finally added, and the reaction was stirred for 2 hours at room temperature under nitrogen protection. The end point of the reaction was detected by TLC (developing agent: dichloromethane: ethyl acetate = 3: 1, chromogen: methanol: acetic acid: concentrated sulfuric acid: anisaldehyde (volume ratio) = 85: 10: 5: 0.5), the reaction was completed , the crude product of com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com