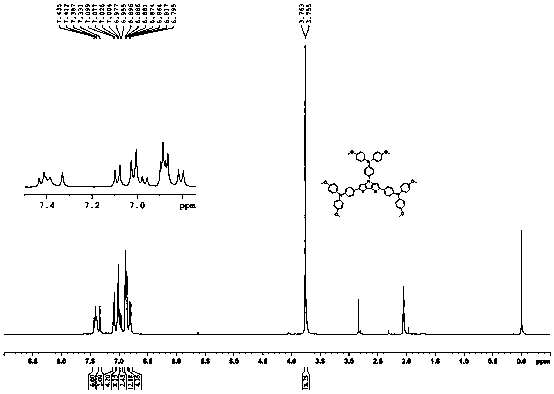
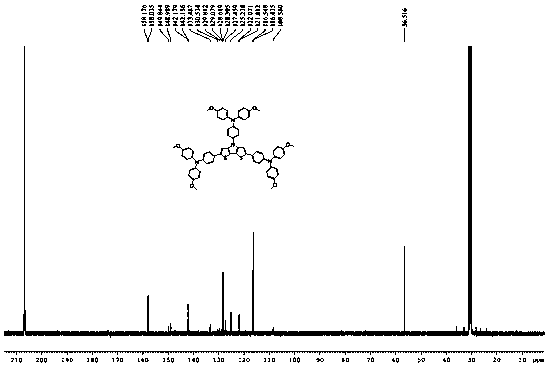
Organic cavity transmission material by using dithiophene pyrrole as core and preparation method and application thereof
A hole transport material, phenopyrrole technology, applied in the field of solar cells, can solve the problems of low efficiency, complex synthesis, poor stability, etc., achieve excellent solubility, simple synthesis route, and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of organic hole transport material
[0024] The synthetic route is as follows
[0025]
[0026] The compound of formula (2) used in this example was synthesized according to literature Liou, G.-S.; , A.; IbrahimDar, M.; Gao, P.; Jankauskas, V.; Jacopin, G.; Kamarauskas, E.; Kazim, S.;Ahmad, S.; Grätzel, M.; . Soc. 2015, 137, 16172-16178 prepared; the compound of formula (1) and other reagents can be obtained commercially.
[0027] Synthesis of the compound of formula (3):
[0028] Under argon protection, 1.05 g of the compound of formula (1), 1.25 g of sodium tert-butoxide, 90 mg of tris(dibenzylideneacetone) dipalladium, 218 mg of 1,1' -Bis(diphenylphosphino)ferrocene, 1.35 g of compound of formula (2) and 15 mL of anhydrous toluene; the reaction system was heated to 110 °C for 8 h and cooled to room temperature, the solvent was distilled off under reduced pressure, and the crude product was passed through the column Analysis (eluent:...
Embodiment 2
[0034] Embodiment 2: the synthesis of organic hole transport material
[0035] Synthesis of the compound of formula (3):
[0036] Under argon protection, 1.05 g of the compound of formula (1), 1.25 g of sodium tert-butoxide, 90 mg of tris(dibenzylideneacetone) dipalladium, 183 mg of 2,2' - Bis-(diphenylphosphino)-1,1'-binaphthalene, 1.35 g of the compound of formula (2) and 15 mL of anhydrous toluene; the reaction system was heated to 110 °C for 8 h and cooled to room temperature, and the solvent was distilled off under reduced pressure , the crude product was purified by column chromatography (eluent: petroleum ether / dichloromethane = 20 / 1~10 / 1) to obtain 1.06 g of the compound of formula (3), a pale yellow solid, with a yield of 68%.
[0037] Synthesis of the compound of formula (4):
[0038] Under argon protection and -78 °C, add 0.8 g of the compound of formula (3) and 10 mL of anhydrous tetrahydrofuran into a 100 mL Schlenk round bottom reaction flask, and then add 1.58...
Embodiment 3
[0042] Embodiment 3: the synthesis of organic hole transport material
[0043] Synthesis of the compound of formula (3):
[0044] Under the protection of argon, 1.05 g of the compound of formula (1), 1.46 g of potassium tert-butoxide, 90 mg of tris(dibenzylideneacetone) dipalladium, 218 mg of 1,1' -Bis(diphenylphosphino)ferrocene, 1.35 g of compound of formula (2) and 15 mL of anhydrous toluene; the reaction system was heated to 110 °C for 8 h and cooled to room temperature, the solvent was distilled off under reduced pressure, and the crude product was passed through the column (eluent: petroleum ether / dichloromethane = 20 / 1~10 / 1) and purified to obtain 59 mg of the compound of formula (3) as a pale yellow solid with a yield of 4%.
[0045] Synthesis of the compound of formula (4):
[0046] Under the protection of argon and at -78 °C, add 0.8 g of the compound of formula (3) and 10 mL of anhydrous tetrahydrofuran into a 100 mL Schlenk round bottom reaction flask, and then a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com