The method of water gas shift reaction
A shift reaction, water gas technology, applied in chemical instruments and methods, catalyst carriers, physical/chemical process catalysts, etc., can solve the problems of easy spontaneous combustion of catalysts, poor start-stop stability, and high reaction temperature, and achieve good technical support, not easy to spontaneous combustion. , the effect of high low temperature conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The preparation method of nano zirconia provided by the invention, the specific steps are as follows:
[0022] a) dissolving the zirconium salt in water to form a solution, preferably at least one of zirconium nitrate, zirconyl nitrate, zirconium oxychloride and zirconium chloride;
[0023] b) adding alkali solution under stirring until the specified pH value is reached, and the pH value at the end point is in the range of 7-12;
[0024] c) Adjust the ambient temperature to 0-150°C, add a reflux device to reflux the precipitate in the alkaline solution for 1-60 hours, and keep the pH value of the mother liquor constant during the entire reflux process;
[0025] d) Filtrating and washing the precipitate until free of impurity ions, drying and roasting. The drying temperature is 60-150°C, the drying time is 4-48 hours, the roasting temperature is 200-800°C, and the roasting time is 1-12 hours.
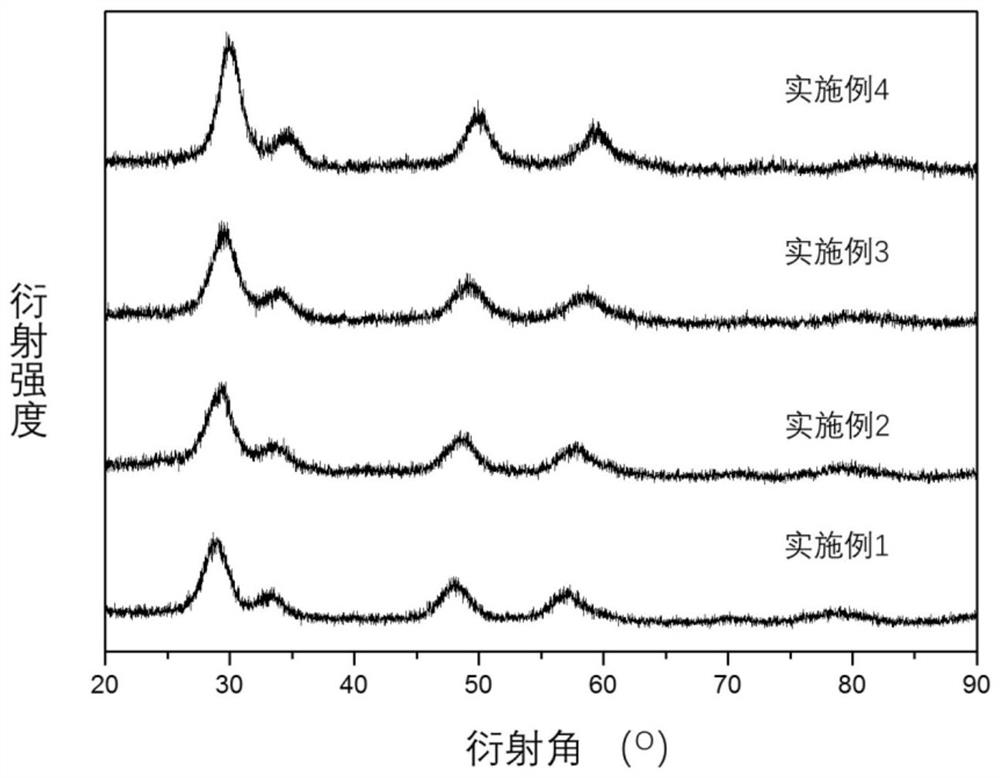
Embodiment 1~4
[0028] Dissolve zirconium nitrate, zirconium oxychloride, oxynitrate and zirconium chloride in deionized water to make 0.5mol L -1 solution, turn on the stirring, add concentrated ammonia water (mass fraction 37%) dropwise at a stirring speed of 300rpm to pH=9, add a reflux device, raise the solution temperature to 100°C, and reflux for 48 hours. Filter and wash the precipitate until there are no impurity ions, dry at 120°C, and bake at 500°C for 4 hours to obtain nano-zirconia. Its structural characterization is shown in Table 1.
Embodiment 5~6
[0030] Dissolve zirconium oxychloride in deionized water to make 0.5mol L -1 solution, turn on the stirring, and add NaOH (2mol L) dropwise at a stirring speed of 300rpm -1 ) and KOH (2mol L -1 ) solution to pH=9, add a reflux device, increase the solution temperature to 100°C, and reflux for 48 hours. Filter and wash the precipitate until there are no impurity ions, dry at 120°C, and bake at 500°C for 4 hours to obtain nano-zirconia. Its structural characterization is shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com