Gentiana macrophylla capsule fingerprint spectrum and application of spectrum to quality control and component analysis
A technology of fingerprint and capsule, applied in the field of drug analysis, to achieve the effect of good analysis and evaluation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
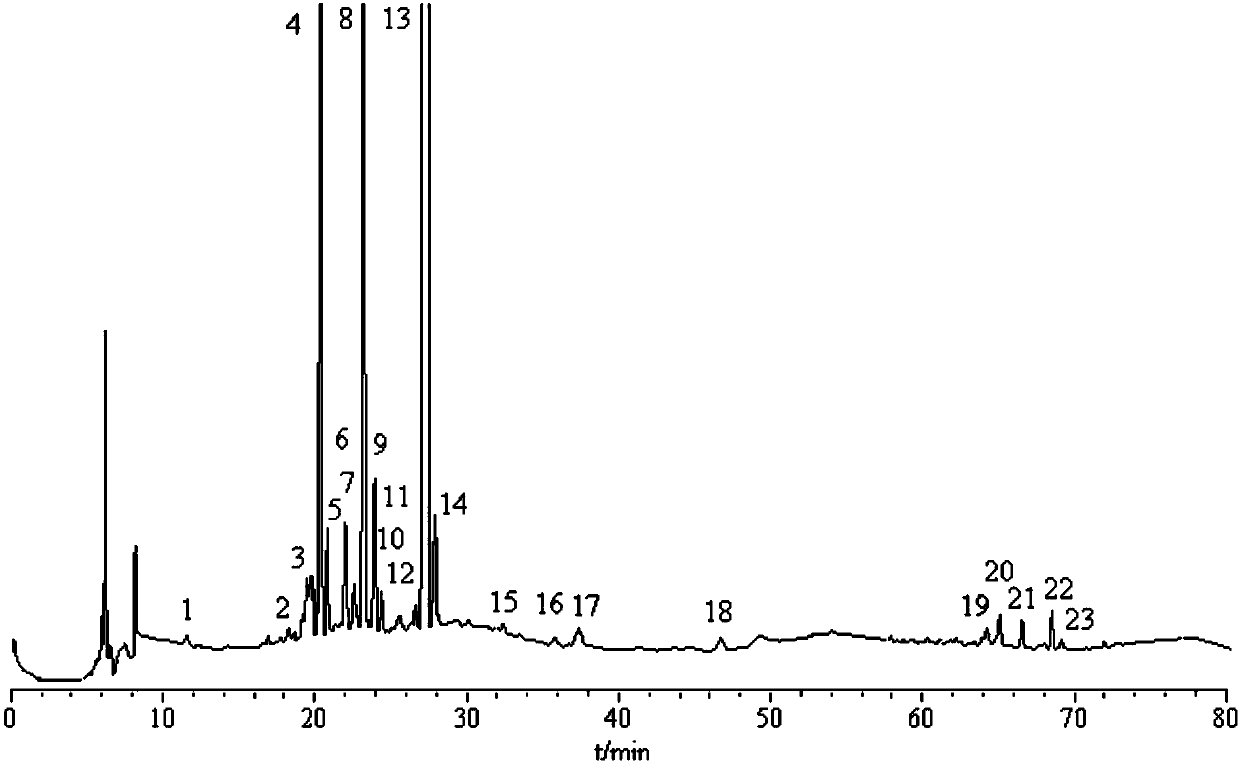
[0040] The establishment of embodiment 1 fingerprint spectrum
[0041] 1 Instruments and reagents
[0042] Shimadzu high performance liquid chromatograph (LC-2010C HT , LC / Labsoluion chromatography workstation), Shimadzu Corporation, Japan; sartorius electronic balance, model CPA225D, Germany Sartorius company; ultrasonic generator, model KQ-5200DE CNC, Kunshan Ultrasonic Instrument Co., Ltd. Acetonitrile, methanol, chromatographic grade, Honeywell Company, USA; Ultrapure water, manufactured by Millipore Water Purifier, USA, Millipore Company, USA; loganic acid (lot number 111865-201403, content of 94.7%), methyl shanzhiside (lot number 111873-201103, with a content of 98.3%), stegopicroside (lot number 110785-201404, with a content of 98.3%), gentiopicroside (with a lot number of 110770-201716, with a content of 99.1%), isoorientin (lot number 111974) -201401, content of 94.0%) and other reference substances, all purchased from China Institute for the Control of Pharmaceuti...
Embodiment 2
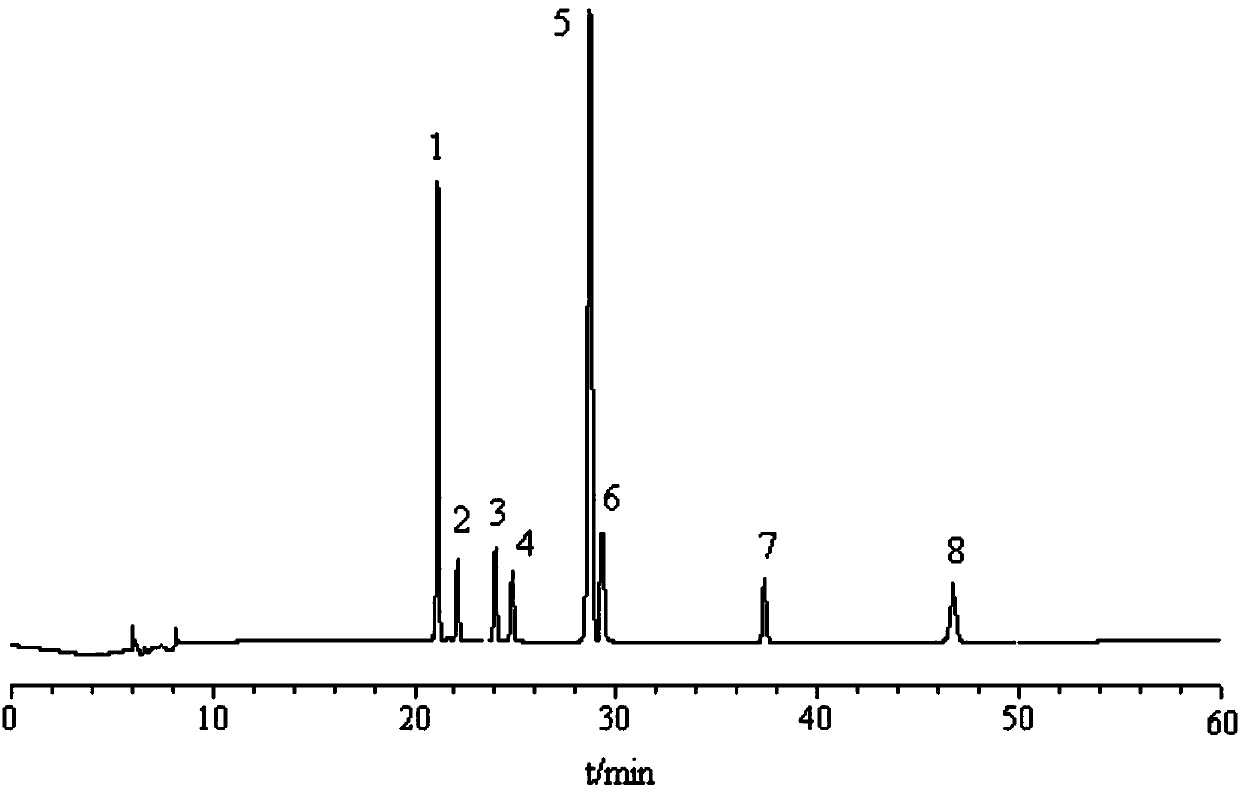
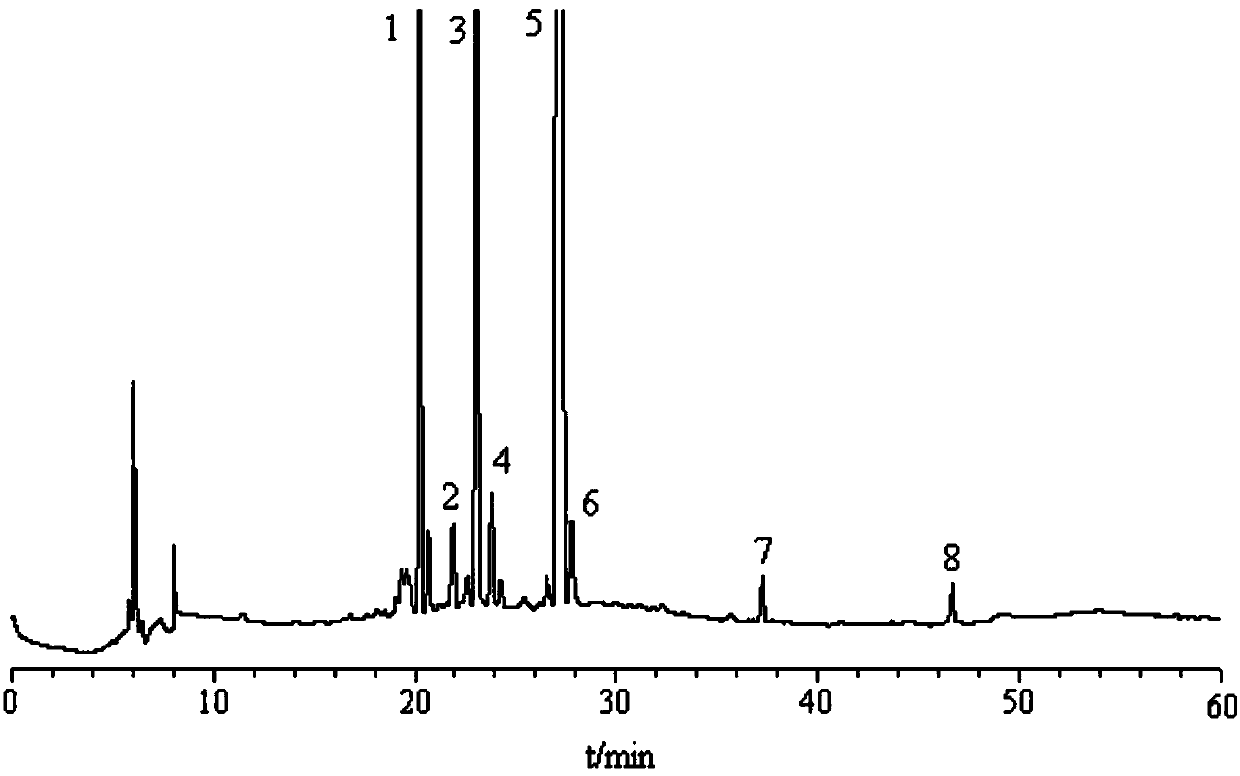
[0052] Embodiment 2 Determination of each component content of Jilong capsule
[0053] 3.1 Chromatographic conditions The recording time is 60min, and the rest are the same as 2.1 of Example 1.
[0054] 3.2 The preparation of the reference substance solution Precisely weighed a certain amount of kaempferol methyl ester, picroside, picroside, isoorientin, isovitexin, loganin, 6′-O -β-D-glucosyl gentiopicroside, gentiopicroside, configured into a reference substance mixed solution, loganinic acid, kaempferol methyl ester, 6′-O-β-D- The concentrations of glucosyl gentiopicroside, steropicrin, gentiopicroside, erythromycin, isoorientin and isovitexin were 2100.00, 320.00, 1500.00, 510.00, 3900.00, 420.00, 220.00, 110μg·mL -1 .
[0055] 3.3 The preparation of the test solution is the same as 2.2.2 in Example 1.
[0056] 3.4 Linear relationship Precisely measure 0.01, 0.05, 0.10, 0.25, 0.50 and 1.00 mL of the reference substance mixture prepared in 3.2 and place them in each 1 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com