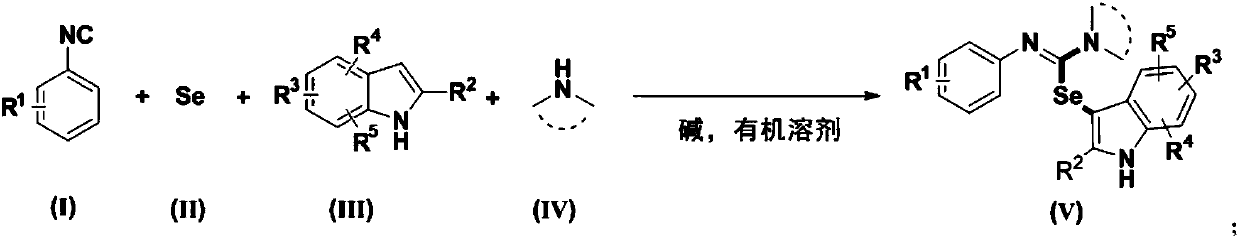
A synthetic method of 3-seleno indoles
A technology of selenoindole and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of dependence, narrow substrate range, low universality, etc., and achieves the effects of simple and mild reaction conditions, easy operation, and cheap and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
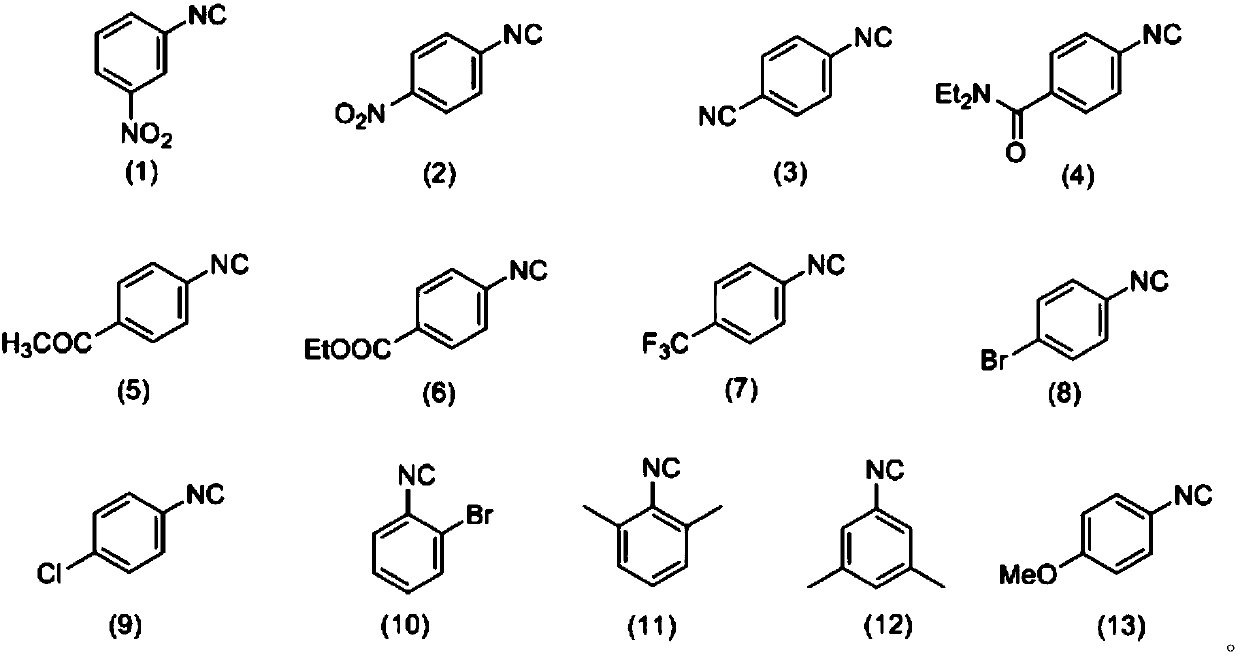
[0038] Synthesis of 1H-indol-3-yl-(Z)-N,N-diethyl-N'-(3-nitrophenyl)carbaimide selenate
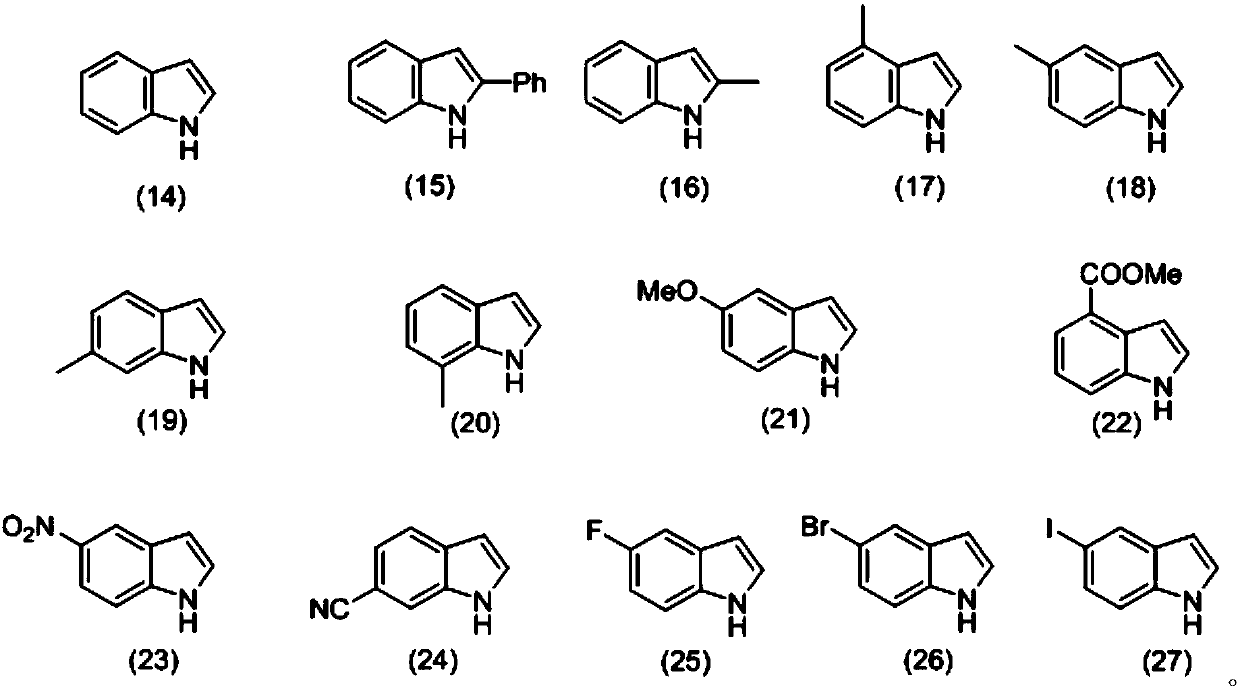
[0039] Weigh 0.3mmol3-nitrophenyl isonitrile (compound corresponding to numbering (1), 0.0444g), 0.36mmol Se (0.0284 g), 0.36mmol indole (compound corresponding to numbering (14), 0.0422g), 0.36 Add mmol diethylamine (compound corresponding to number (28), 0.0263g, 38μL), 0.6mmol cesium carbonate (0.2117g) in a 20mL test tube reaction tube, add 2mL tetrahydrofuran as solvent, and stir at 40°C for 12 hours; After the reaction, the reaction solution was spin-dried, loaded and separated by column chromatography (column chromatography separation conditions: the stationary phase was 300-400 mesh silica gel powder, and the mobile phase was ethyl acetate (A) and petroleum ether (B). , the mobile phase change program (A:B) is 1:8), and 0.0810 g of the reaction product was obtained.
[0040] The above-mentioned reaction product is characterized, and the result is:
[0041] 1 H NMR (400MHz, CDCl...
Embodiment 2
[0044] Synthesis of 1H-indol-3-yl-(Z)-N,N-diethyl-N'-(3-nitrophenyl)carbaimide selenate
[0045] Weigh 0.3mmol3-nitrophenyl isonitrile (compound corresponding to numbering (1), 0.0444g), 0.36mmol Se (0.0284 g), 0.36mmol indole (compound corresponding to numbering (14), 0.0422g), 0.36 mmol diethylamine (number (28) corresponding compound, 0.0263g, 38μL), 0.6mmol cesium carbonate (0.2117g) and 0.06mmol2,2,6,6-tetramethylpiperidine oxide (0.0094g) in In a 20mL test tube reaction tube, add 2mL tetrahydrofuran as a solvent, and stir and react at 40°C for 16 hours; ~400 mesh silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) is 1:8), and 0.1171g of the reaction product is obtained.
[0046] The above-mentioned reaction product is characterized, and the result is:
[0047] 1 H NMR (400MHz, CDCl 3) δ = 8.32 (s, 1H), 7.40–7.31 (m, 2H), 7.20–7.07 (m, 4H), 6.87 (t, J = 8.0Hz, 1H), 6.63 (dd ,J=7.8,1.2Hz,1H),6.58(d,J=...
Embodiment 3
[0050] Synthesis of 1H-indol-3-yl-(Z)-N,N-diethyl-N'-(4-nitrophenyl)carbaimide selenate
[0051] Weigh 0.3mmol 4-nitrophenyl isonitrile (compound corresponding to numbering (2), 0.0444g), 0.36mmol Se (0.0284 g), 0.36mmol indole (compound corresponding to numbering (14), 0.0422g), 0.36 mmol diethylamine (number (28) corresponding compound, 0.0263g, 38μL), 0.6mmol cesium carbonate (0.2117g) and 0.06mmol2,2,6,6-tetramethylpiperidine oxide (0.0094g) in In a 20mL test tube reaction tube, add 2mL tetrahydrofuran as a solvent, and stir and react at 40°C for 16 hours; ~400 mesh silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) is 1:8), and 0.0679g of the reaction product is obtained.
[0052] The above-mentioned reaction product is characterized, and the results are as follows:
[0053] 1 H NMR (400MHz, DMSO-d 6 )δ=11.29(s,1H),7.68–7.62(m,2H),7.27(dd,J=18.9,7.6 Hz,2H),7.09–7.01(m,2H),6.86(d,J=2.7Hz ,1H),6.33–6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com