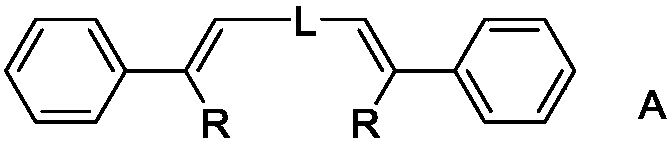
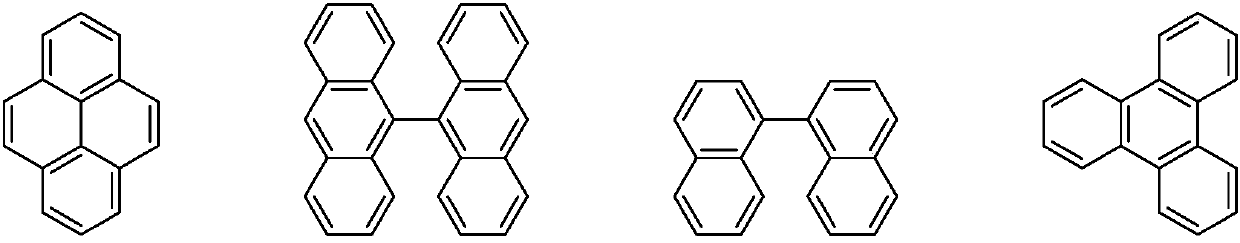
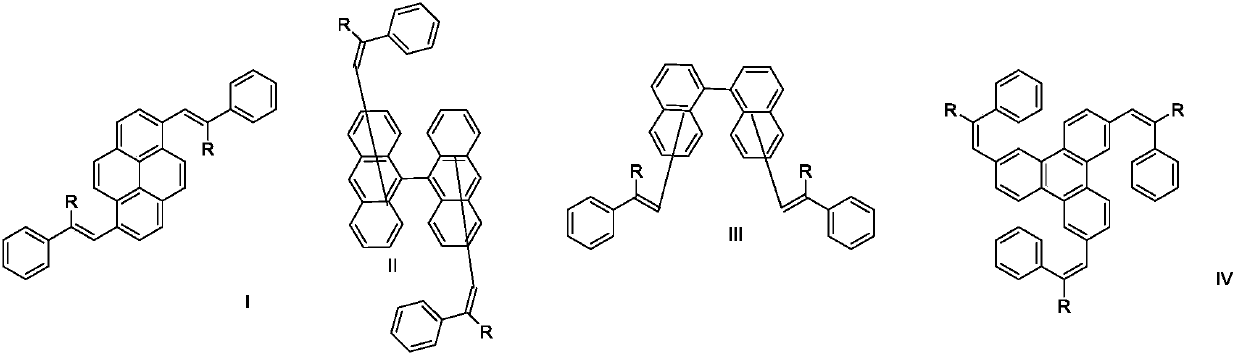
Organic electroluminescence material and organic light-emitting diode
A technology for electroluminescent materials and organic light-emitting devices, which is applied in the directions of organic light-emitting devices, light-emitting materials, and materials of organic semiconductor devices, etc., can solve the problems of low performance and large energy difference of blue light-emitting materials, and achieve the effect of prolonging life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: 1 synthesis of compound
[0047]
[0048] Step1. Add 100mmol formyl chloride to anhydrous THF, add a THF solution of phenylmagnesium bromide dropwise, and react the mixture at room temperature for 2h. Ice water was added, stirred, and the reaction solution was extracted with dichloromethane and dried. The obtained crude product was subjected to column chromatography to obtain 80 mmol of product 1-1. Yield 80%
[0049] Step2. Under the protection of nitrogen, 96mmol of triphenylphosphine bromide was dissolved by adding an appropriate amount of anhydrous THF, then cooled to 0°C, and 1.2 equivalents of potassium tert-butoxide was added, and the reaction was kept for 1 hour, and 1-1 80mmol was quickly added dropwise. The THF solution was slowly warmed to room temperature and reacted overnight. After the reaction was completed, most of the reaction solution was concentrated, then passed through a thin Celite column, and continued to concentrate to dryness...
Embodiment 2
[0051] Embodiment 2: 2 synthesis of compound
[0052]
[0053] Step1. Add 100mmol formyl chloride to anhydrous THF, add a THF solution of phenylmagnesium bromide dropwise, and react the mixture at room temperature for 2h. Ice water was added, stirred, and the reaction solution was extracted with dichloromethane and dried. The obtained crude product was subjected to column chromatography to obtain 80 mmol of product 1-1. Yield 80%
[0054]Step2. Under the protection of nitrogen, 96mmol of triphenylphosphine bromide was dissolved by adding an appropriate amount of anhydrous THF, then cooled to 0°C, and 1.2 equivalents of potassium tert-butoxide was added, and the reaction was kept for 1 hour, and 1-1 80mmol was quickly added dropwise. The THF solution was slowly warmed to room temperature and reacted overnight. After the reaction was completed, most of the reaction solution was concentrated, then passed through a thin Celite column, and continued to concentrate to dryness ...
Embodiment 3
[0056] Embodiment 3: 3 synthesis of compound
[0057]
[0058] Step1. Add 100mmol formyl chloride to anhydrous THF, add a THF solution of phenylmagnesium bromide dropwise, and react the mixture at room temperature for 2h. Ice water was added, stirred, and the reaction solution was extracted with dichloromethane and dried. The obtained crude product was subjected to column chromatography to obtain 80 mmol of product 1-1. Yield 80%
[0059] Step2. Under the protection of nitrogen, 96mmol of triphenylphosphine bromide was dissolved by adding an appropriate amount of anhydrous THF, then cooled to 0°C, and 1.2 equivalents of potassium tert-butoxide was added, and the reaction was kept for 1 hour, and 1-1 80mmol was quickly added dropwise. The THF solution was slowly warmed to room temperature and reacted overnight. After the reaction was completed, most of the reaction solution was concentrated, then passed through a thin Celite column, and continued to concentrate to dryness...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com