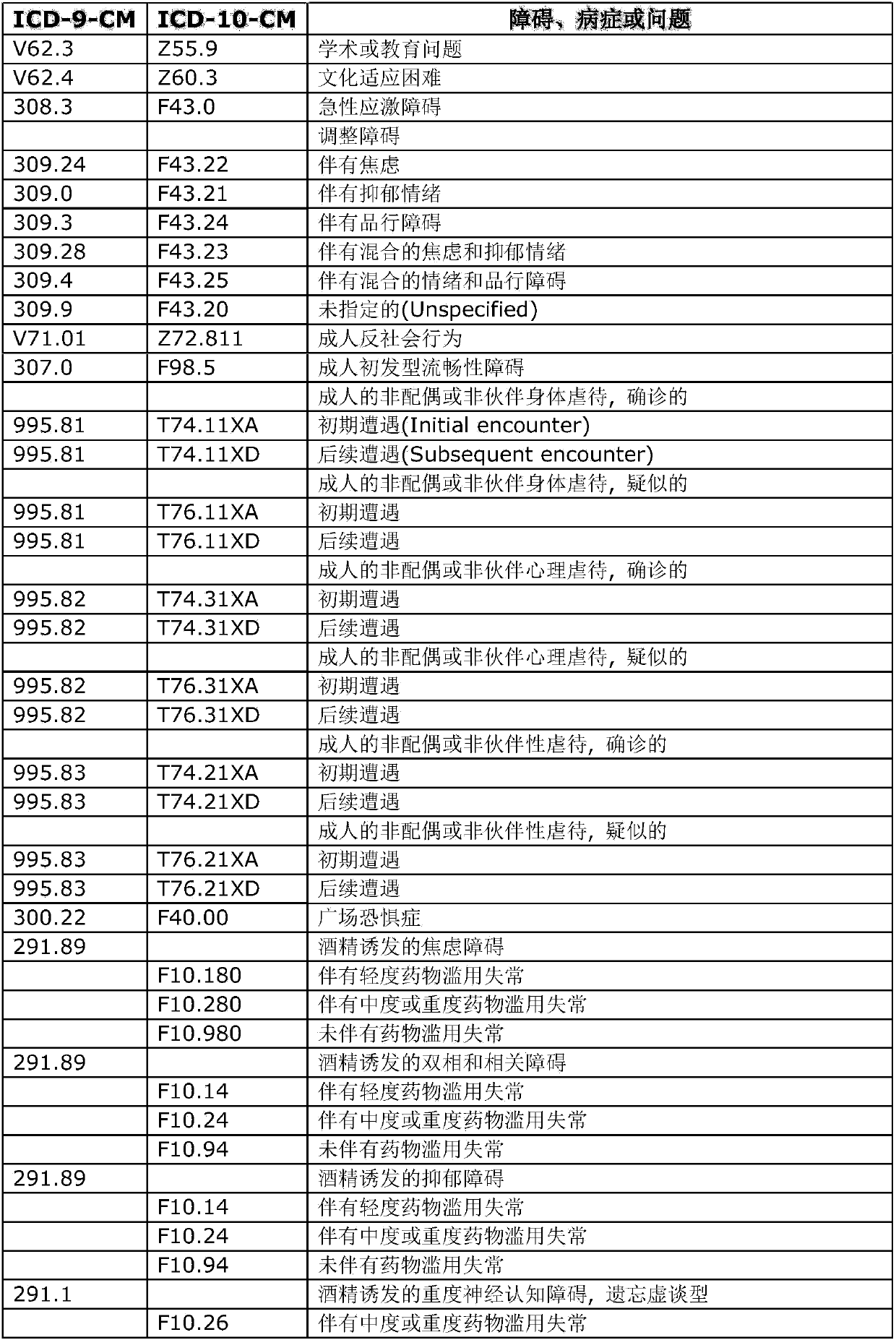
Sublingual administration of riluzole
A technology of riluzole and drug administration, which is applied in the field of sublingual administration of riluzole, to achieve the effect of reducing side effects and weakening abnormal liver function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] A 51-year-old man received four doses of riluzole.
[0089] (1) Riluzole in a normal oral administration form was used as a comparator. A standard 50 mg riluzole tablet (not a tablet of the present invention) was crushed and administered into the oral cavity for 40 seconds to allow transmucosal and / or oral absorption. This is not sublingual or buccal administration. No acute or chronic effects on the neuropsychiatric field. More specifically, there was no effect on mood, anxiety or behaviour. Prominent oral numbness was noted. Within the first minute, numbness spreads throughout the mouth, including the back of the tongue and lips, causing perioral paresthesias. The effect is moderate and peaks within 4 minutes. The effect lasts for up to 80 minutes. The effect started to decline after 15 minutes, was considered mild after 40 minutes, and minimal after 80 minutes. All effects are limited to the aforementioned local oral sensations.
[0090] (2) In addition, the ...
Embodiment 2
[0096] A 43-year-old man took riluzole twice.
[0097] (1) Oral administration: about 50 mg of uncrushed riluzole tablets (not the formulation of the present invention) were placed on the tongue of the subject. But no psychotropic effects were reported after immediate application, and subjects reported numbness in the area of topical application that spread rapidly throughout the oral cavity. The numbness lasted 20 minutes. No sense of mood or behavior was reported. The numbness is strong and annoying.
[0098] (2) Sublingual administration: about 20 mg of riluzole sublingual preparation was placed under the tongue of the subject for about 30 seconds. Within about 20 minutes, subjects reported onset of beneficial psychoactive effects, including feelings of relaxation, calm, and less anxiety. Subjects also reported feeling alert. These psychoactive effects or sensations lasted about 90 minutes. The subject noted that his gastrointestinal tract felt "calmed" and his prev...
Embodiment 3
[0101] A 50-year-old man took a sublingual formulation of riluzole.
[0102] A sublingual formulation of about 5 mg of riluzole was placed under the subject's tongue for approximately 20 seconds until the formulation was completely dissolved. Again, in other subjects who received sublingual administration, unexpected psychoactive effects occurred shortly after dosing. Within 20 to 30 minutes, the subject reported the onset of beneficial psychoactive effects, and he reported a feeling of relaxation and calm.
[0103] About 7 minutes after dosing, the subject reported numbness in his mouth and the top of his tongue, which peaked at about 7 minutes and then completely disappeared about 21 minutes later.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com