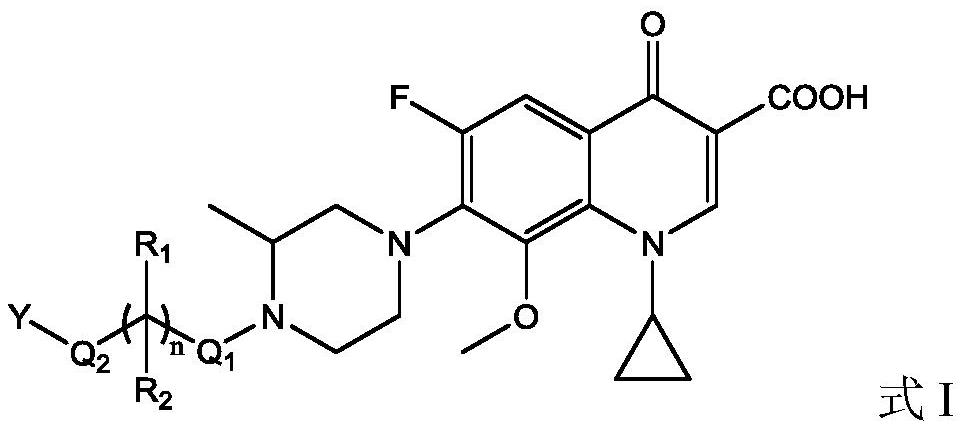
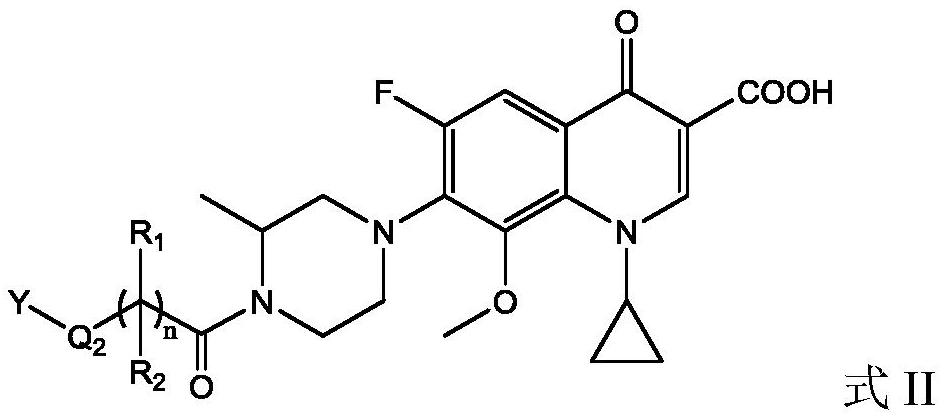
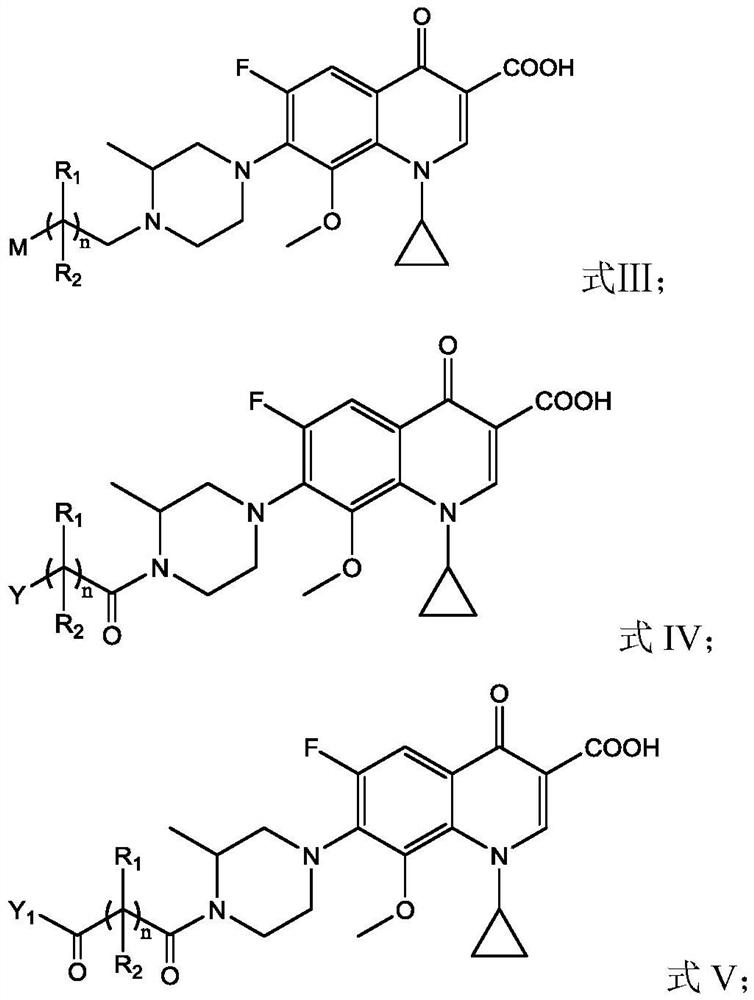
Gatifloxacin derivative and its preparation method and use
A compound and selected technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve problems such as abnormal blood sugar, achieve enhanced antibacterial activity, reduced toxic and side effects, and good antibacterial activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0079] Preparation Example 1: Preparation of IM1
[0080] Add GAT 5mmol, DCM 15mL, NaHCO 3 7.5 mmol, after stirring evenly, slowly add 2.5 mmol of solid phosgene in 5 mL of dichloromethane (DCM) dropwise under ice bath. After the dropwise addition, move to room temperature to stir the reaction, and monitor the reaction progress by TLC. After the reaction was completed, 10 mL of ice-cold saturated NaCl solution was added, and 2N HCl solution was added to adjust the pH value to 4-5, and the liquids were separated. Saturated NaCl solution 10mL washed, separated, organic phase anhydrous NaCl 2 SO 4 After drying, the solvent was removed by rotary evaporation to obtain the pure product IM1.
preparation example 2
[0081] Preparation Example 2: Preparation of IM2
[0082] Into a 100mL round bottom flask, add raw materials GAT 1mmol, NaHCO 3 2.5mmol, DCM 5mL, add dropwise a mixed solution of chloroacetyl chloride 2mmol and DCM 2mL under ice-cooling. After dropping, the reaction was continuously stirred under ice bath, and the reaction progress was monitored by TLC. After the reaction, add a little ice-cold saturated NaCl solution to dissolve the solid, adjust the pH to 4-5 with ice-cold 2N HCl solution, stir evenly, transfer to a separatory funnel for liquid separation, extract twice with DCM, combine the organic phases, and wash with saturated NaCl solution. Water Na 2 SO 4 Dry and remove the solvent by rotary evaporation. The pure product was obtained by recrystallization or column chromatography, dried and weighed to obtain IM2. Preparation Example 3: Preparation of IM3
preparation example 3
[0083] Into a 100mL round bottom flask, add raw materials GAT 1mmol, NaHCO 3 2.5mmol, DCM 5mL, add dropwise a mixed solution of chloropropionyl chloride 2mmol and DCM 2mL under ice-cooling. After dropping, the reaction was continuously stirred under ice bath, and the reaction progress was monitored by TLC. After the reaction, add a little ice-cold saturated NaCl solution to dissolve the solid, adjust the pH to 4-5 with ice-cold 2N HCl solution, stir evenly, transfer to a separatory funnel for liquid separation, extract twice with DCM, combine the organic phases, and wash with saturated NaCl solution. Water Na 2 SO 4 Dry and remove the solvent by rotary evaporation. The pure product was obtained by recrystallization or column chromatography, dried and weighed to obtain IM3. Preparation Example 4: Preparation of IM4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com