Ibrutinib synthesis method
A synthesis method, the technology of ibrutinib, applied in the field of drug synthesis, can solve the problems of ibrutinib quality research and quality control, increase the difficulty of ibrutinib purification, unstable chemical properties, etc., and achieve quality Easy to control, quality controllable, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] This embodiment provides a synthetic method of ibrutinib, which includes:
[0017] Compound M and the compound of formula I undergo an acylation reaction in the presence of a base, and its reaction formula is as follows:
[0018]
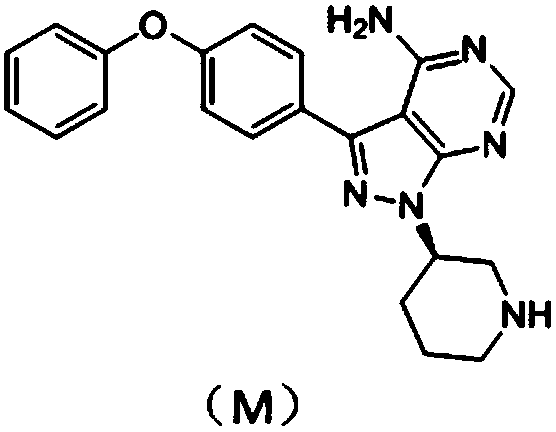
[0019] Wherein, compound M can be bought, also can choose to adopt following route synthetically to obtain:
[0020]
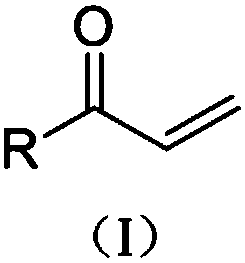
[0021] The general structural formula of formula I compound is:
[0022]
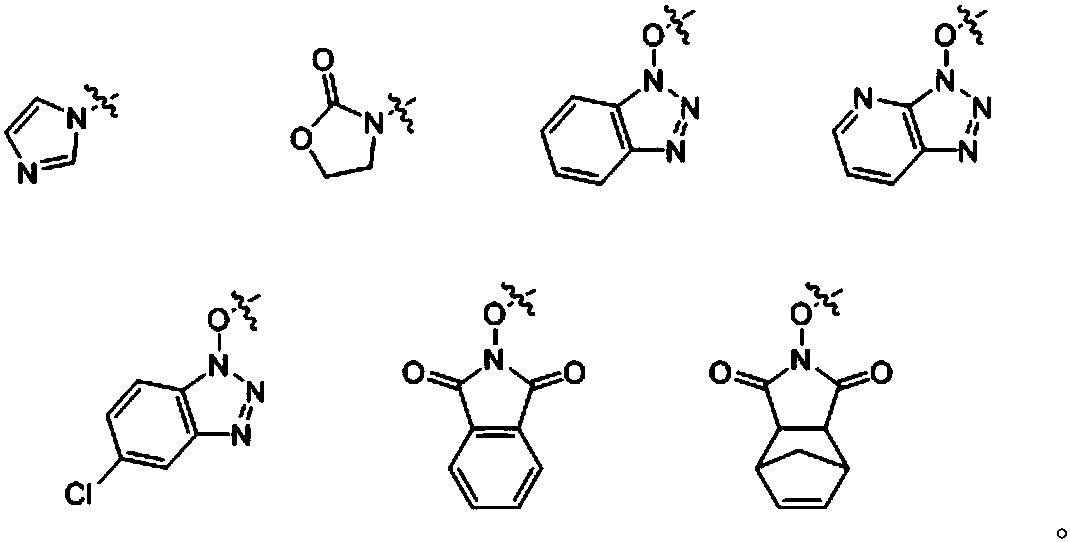
[0023] Wherein, R is selected from:
[0024]
[0025] Optionally, compounds of formula I include the following compounds:
[0026]
[0027] Further, the base added in the above-mentioned acylation reaction includes N,N-diisopropylethylamine, triethylamine, N-methylmorpholine and 1,8-diazabicycloundec-7 -at least one of alkenes. Optionally, the equivalent of the base added is 2-5eq., or 2.5-3.5eq., or 3eq.
[0028] Further, the temperature of the reaction liquid when the base is added in the above acylation reaction is -5-10°C, or 0-...
Embodiment 1-1
[0044] This embodiment provides a synthetic method of ibrutinib, which includes:
[0045] Step S1: the synthesis of formula (I-a) compound, synthetic route is as follows:
[0046]
[0047] Add acrylic acid (100g, 1.39mol, 1.00eq.) to a 2L reaction flask containing 1L of dichloromethane, add carbonyldiimidazole (292.53g, 1.81mol, 1.30eq.) in batches, and stir at 20-30°C for 2 hours , Gas chromatography (GC) detects that the acrylic acid has basically reacted completely. Add 0.5L water to the reaction system to stir and separate the liquid, separate the water phase, repeat 3 times, dichloromethane solution is concentrated under reduced pressure, and the obtained solid is recrystallized with methyl tert-butyl ether After drying, off-white solid 1-(1H-imidazol-1-yl)prop-2-en-1-one (151.07g, 1.23mol) was obtained, which was the compound of formula (I-a), with a yield of 88.50%, HPLC purity 99.55%.
[0048] Step S2: Synthesis of ibrutinib, the synthetic route is as follows:
...
Embodiment 1-2
[0052] This example provides a synthetic method of ibrutinib, the synthetic route of which is consistent with that of Example 1, except that step S2:
[0053]
[0054](R)-3-(4-phenoxyphenyl)-1-(piperidin-3-yl)-1H-pyrazol[3,4-d]pyrimidin-4-amine (200g, 0.52mol, 1.00eq.) and 1-(1H-imidazol-1-yl)prop-2-en-1-one (76.20g, 0.62mol, 1.20eq.) were added to a 5L reaction flask containing 3L dichloromethane, Add 1,8-diazabicycloundec-7-ene (197.77g, 1.30mol, 2.50eq.) dropwise at -5-0°C, raise the temperature to 15-25°C and stir for 1 hour, HPLC detects that the reaction is complete, Add 1L of dichloromethane, wash the organic phase twice with 2L of dilute phosphoric acid aqueous solution, wash with 2L of dilute sodium carbonate aqueous solution, wash with 2L of purified water, and concentrate under reduced pressure. The mixed solvent was crystallized, and after drying, ibrutinib (167.39 g, 0.38 mol) was obtained with a yield of 78.85%, a HPLC purity of 99.81%, and a maximum of 0.03%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com