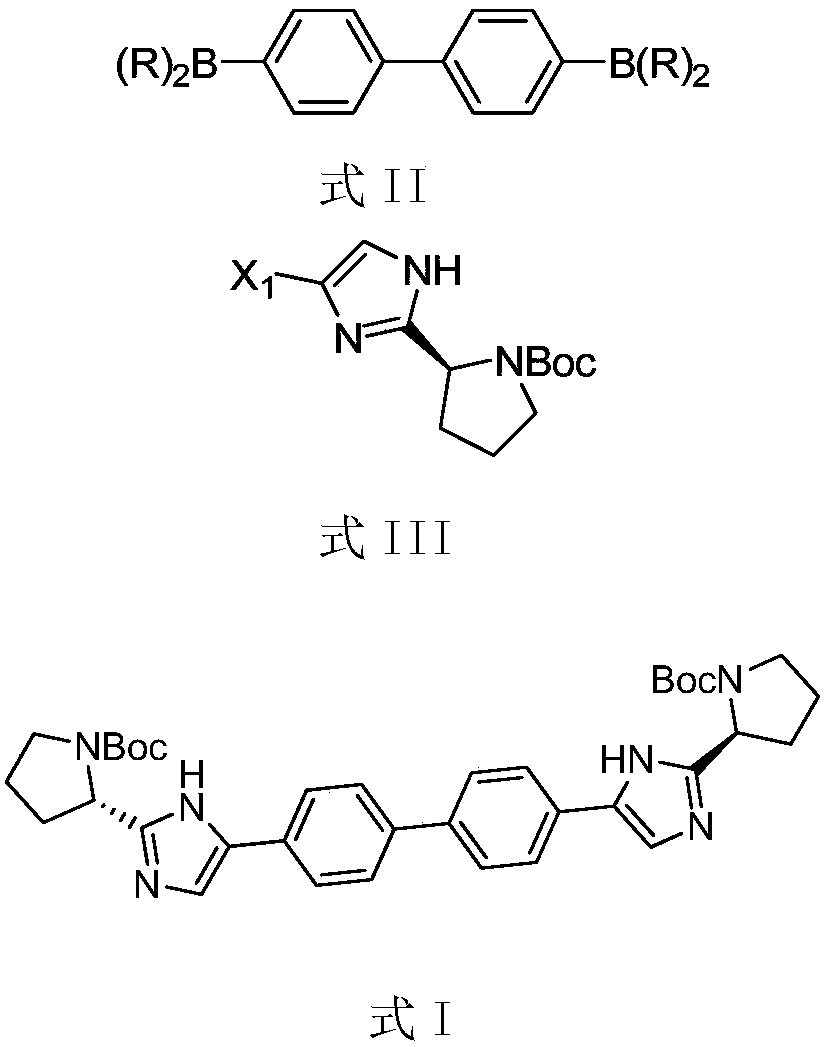
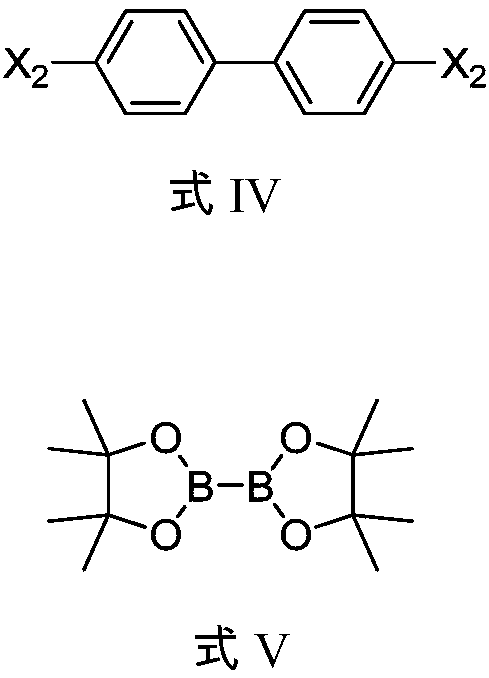
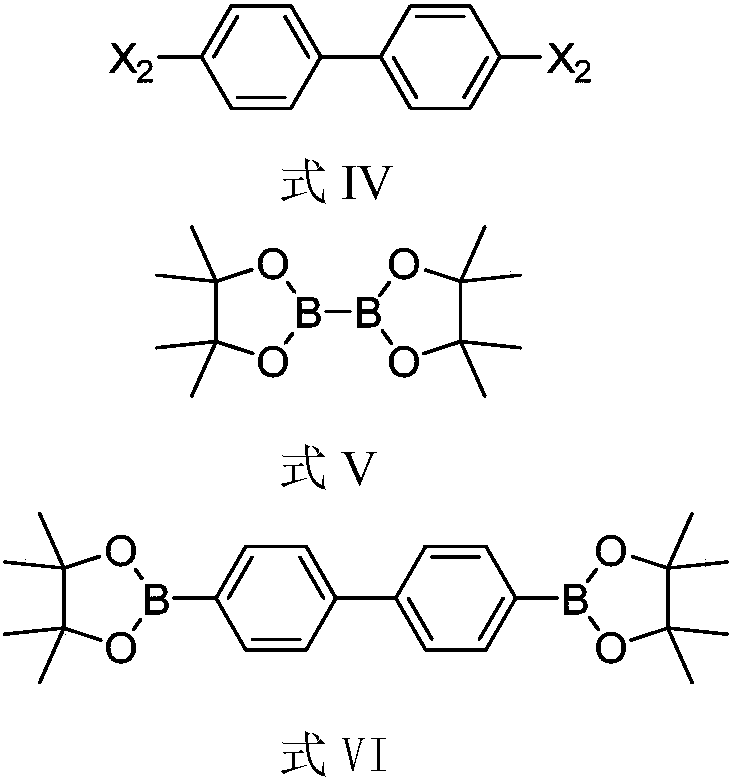
Method for preparing compound
A technology of compounds and alkaline conditions, applied in the field of medicine, to achieve the effects of increased efficiency, high yield, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055] The preparation process is as follows:
[0056] Add DSV206 (500mg), pinacol borate (854mg), potassium acetate (629mg), PdCl 2 (P(tBu) 2 Ph) 2 (40mg), ethylene glycol dimethyl ether (DME) (5ml), nitrogen replacement 3 times, react at 80°C for 5h, cool down, add DSV205 (1.45g), potassium carbonate (538mg), PdCl 2 (P(tBu) 2 Ph) 2 (100mg), water (1ml), DME (10ml), nitrogen replacement 3 times, react at 80°C for 18h. Add 10ml of ethyl acetate and 10ml of water, stir for 15min, separate the liquids, add 10ml of ethyl acetate to the aqueous phase, stir for 15min, separate the liquids, combine the organic phases, concentrate the organic phases under reduced pressure to obtain a yellow turbid oily liquid, and separate by column chromatography to obtain 620 mg off-white solid DSV103, yield 62.5%, purity 98.2%.
[0057] ESI-MS[m+H]+:625.3499; 1H NMR(400MHz,DMSO-d6)δ13.00-11.00(s,2H),7.90-7.75(m,4H),7.75-7.60(m,4H), 7.60-7.30(s,2H),4.92-4.72(m,2H),3.65-3.49(m,2H)...
Embodiment 2
[0059]
[0060] The preparation process is as follows:
[0061] Add DSV206 (1.00g), pinacol borate (1.86g), potassium acetate (1.72g), PdCl 2 (dppf) (0.11g), 1,4-dioxane (10ml), nitrogen replacement 3 times, reacted at 100°C for 22h, lowered the temperature, separated by column chromatography to obtain 1.22g solid, added DSV208 (2.00g), carbonic acid Sodium hydrogen (1.26g), PdCl2 (P(tBu)2Ph)2 (94mg), water (3ml), Dioxane (12ml), nitrogen replacement 3 times, 80 ℃ for 24h. The temperature was lowered to crystallize and separated by column chromatography to obtain 1.50 g of off-white solid DSV103 with a yield of 75.3% and a purity of 95.4%.
Embodiment 3
[0063]
[0064] The preparation process is as follows:
[0065] Add DSV210 (270mg), pinacol diborate (371mg), potassium phenate (KOPh) (351mg), Pd(PPh3)4 (80mg), toluene (10ml) into a 50ML three-necked flask, and replace with nitrogen for 3 Once, react at 90°C for 10h, cool down, add DSV208 (500mg), sodium carbonate aqueous solution (1M, 2.7ml), PdCl 2 (dppf) (50mg), toluene (10ml), nitrogen replacement 3 times, react at 90°C for 16h. The temperature was lowered for crystallization, and column chromatography was used for separation to obtain 337 mg of off-white solid DSV103 with a yield of 80.2% and a purity of 96.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com