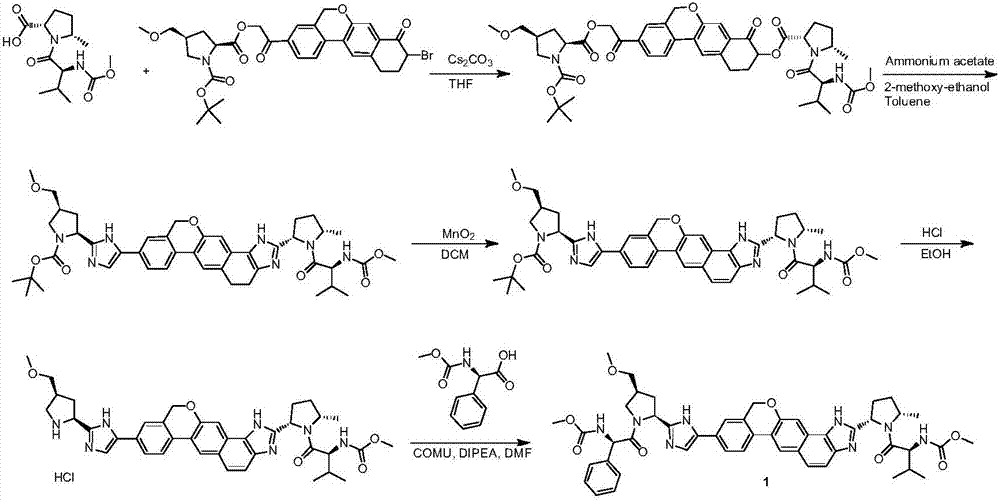
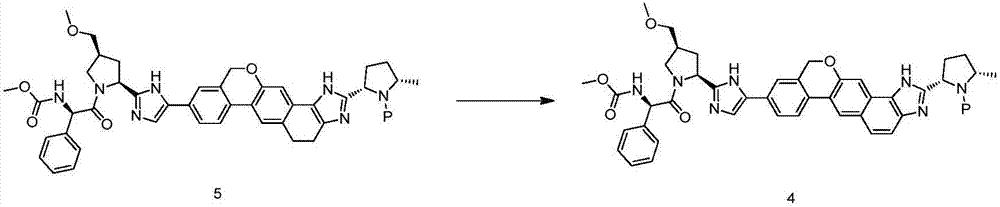
Velpatasvir intermediate as well as preparation method and application thereof
A reaction and time technology, applied in chemical instruments and methods, bulk chemical production, organic chemistry, etc., can solve problems such as high production costs, failure to meet API standards, and unsuitability for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0218] Example 1: Preparation of compound 10 (L is Br, P is tert-butoxycarbonyl, namely Boc)
[0219]
[0220] Compound 11 (2kg, 3.18mol, 1eq) was dissolved in methanol, and a mass concentration of 37% concentrated hydrochloric acid (1.33L, 5eq) was added (the mass concentration refers to the percentage of the mass of hydrogen chloride in the total mass of the hydrochloric acid solution), heated Reflux (60°C~65°C), react for 2-6 hours, TLC shows complete reaction, drop to room temperature, add methyl tert-butyl ether slowly, after the dropwise addition, stir at 0-10°C for 2-5 hours, filter , the solid was suspended in dichloromethane, saturated sodium bicarbonate was added, stirred for 1 hour, filtered, the organic phase was washed with water and saturated sodium chloride successively, concentrated to dryness, and 1.52kg of compound 10 was obtained, the yield was 90.0%, and the HPLC purity was 98.23% .
Embodiment 2
[0221] The preparation of embodiment 2 compound 14 (P is Boc)
[0222]
[0223] Compound 25 (1.96kg, 5.29mol, 1.0eq) and compound 24 (1.37kg, 1.05eq) were dissolved in N, N-dimethylformamide (DMF), potassium carbonate (730.8g, 1.05eq) was added, nitrogen Vacuum replaced three times, heated to 20-40°C and stirred for 3 hours. TLC showed complete reaction of compound 25. Reduce to 5-10°C, add water dropwise, stir at 5-10°C for 2-3 hours, filter, wash the filter cake with water, and dry to obtain 2.85kg of compound 14 with a yield of 98.0% and an HPLC purity of 98.56%.
Embodiment 3
[0224] Preparation of Example 3 Compound 13 (P is Boc, i.e. tert-butoxycarbonyl)
[0225]
[0226] Compound 14 (2.85kg, 5.18mol, 1eq) was dissolved in isopropyl acetate, added isopropyl acetate / hydrogen chloride (1.33L, 5eq), heated to reflux (60°C-65°C), reacted for 2-6 hours, TLC Show complete reaction, lower to room temperature, slowly add methyl tert-butyl ether dropwise, stir at 0-10°C for 2-5 hours after dropwise addition, filter, suspend the solid in dichloromethane, add saturated sodium bicarbonate, stir After filtering for 1 hour, the organic phase was washed with water and saturated sodium chloride successively, and concentrated to dryness to obtain 2.21 kg of compound 13 with a yield of 95.0% and a purity of 98.86% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com