Synthesis method of <18>F-labeled amino acid polypeptide drugs and kit
A drug synthesis and amino acid technology, applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of radiation safety protection risks, difficult automatic industrial production, etc., and achieve the promotion of development, huge social benefits and social benefits. Benefit, the effect of high-dose industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 118
[0048] Example 1 18 Fully automatic industrial synthesis of F-AlF-NOTA-Glu injection
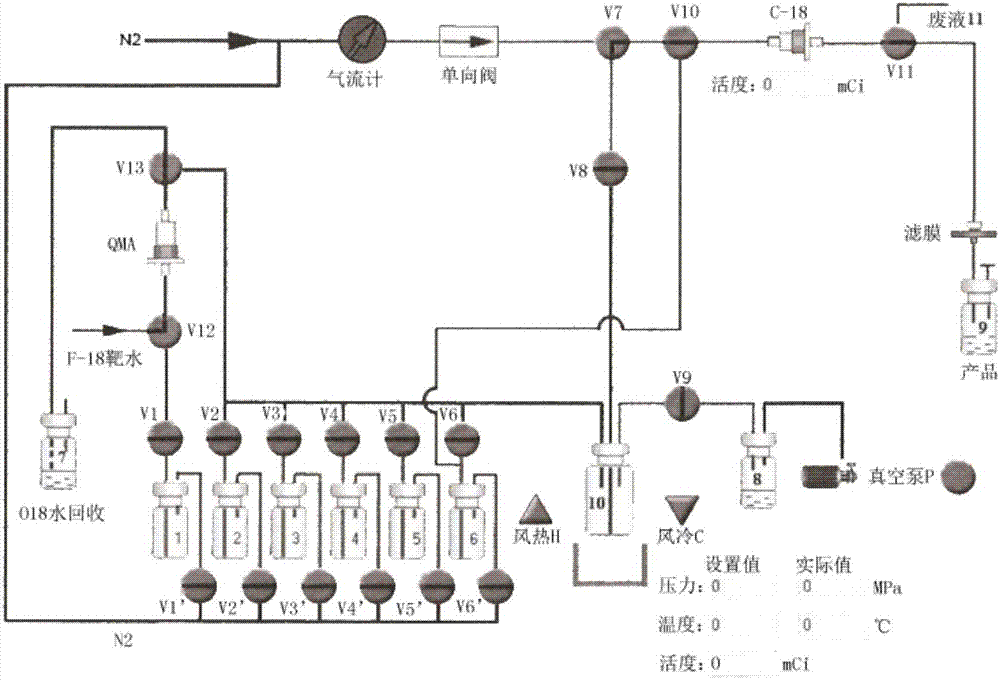
[0049] Kit composition: 0.4-0.5mL physiological saline (bottle 1), 200-300μg precursor NOTA-Glu+2mM 24μL AlCl 3 (bottle 2), 0.8-1.2mL pH4-5 acidic acetonitrile solution (bottle 2') (bottle 2' is in the kit, but in figure 1 Not shown in , the solution from vial 2' was added to vial 2 before synthesis), 15 mL of water for injection (bottle 3), 10 mL of water for injection (bottle 4), 1.5 mL of ethanol (bottle 5) and 18 mL of 0.57 mM ascorbic acid containing Normal saline (bottle 6). Auxiliary materials include: SEP-PAK C18 separation column, SEP-PAK QMA column, column pretreatment solvent or solution (ethanol, water for injection, 8.4% sodium bicarbonate solution), syringe and needle, receiving bottle, import and export without Bacterial membrane. SEP-PAK QMA cartridges can also be replaced by other anion cartridges; physiological saline can be replaced by other inorganic salt solutions; a...
Embodiment 218
[0052] Example 2 18 Fully automatic industrial synthesis of F-AlF-NOTA-NOC injection
[0053] Kit composition: 0.4-0.5mL physiological saline (bottle 1), 200-300μg precursor NOTA-NOC+2mM 24μL AlCl 3 (bottle 2), 0.8-1.2mL pH4-5 acidic acetonitrile solution (bottle 2'), 15mL normal saline (bottle 3), 10mL normal saline (bottle 4), 1.5mL ethanol (bottle 5) and 18mL containing 0.57mM ascorbic acid Normal saline (bottle 6). Auxiliary materials include: HLB small column, SEP-PAK QMA small column, small column pretreatment solvent or solution (ethanol, normal saline, water for injection, 8.4% sodium bicarbonate solution), syringe and needle, receiving bottle, sterile import and export filter membrane. SEP-PAK QMA cartridges can also be replaced by other anion cartridges; physiological saline can be replaced by other inorganic salt solutions; acetonitrile can be replaced by dimethyl sulfoxide, ethanol, and methanol; HLB cartridges can also be separated by SEP-PAK C18 small column ...
Embodiment 318
[0056] Example 3 18 Fully automatic industrial synthesis of F-AlF-NOTA-PEG3-β-Glu-RGD2 injection
[0057] Kit composition: 0.4-0.5mL normal saline (bottle 1), 200-300μg precursor NOTA-PEG3-β-Glu-RGD2+2mM 24μL AlCl 3 (bottle 2), 0.8-1.2mL pH4-5 acidic acetonitrile solution (bottle 2'), 15mL water for injection (bottle 3), 10mL water for injection (bottle 4), 1.5mL ethanol (bottle 5) and 18mL containing 0.57mM ascorbic acid Normal saline (bottle 6). Auxiliary materials include: SEP-PAK C18 separation column, SEP-PAK QMA column, column pretreatment solvent or solution (ethanol, water for injection, 8.4% sodium bicarbonate solution), syringe and needle, receiving bottle, import and export without Bacterial membrane. SEP-PAK QMA cartridges can also be replaced by other anion cartridges; physiological saline can be replaced by other inorganic salt solutions; acetonitrile can be replaced by dimethyl sulfoxide, ethanol, and methanol; SEP-PAK C18 cartridges can also be replaced by H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com