Peripheral drug eluting stent and preparation and application thereof
A technology for eluting stents and drugs, applied in medical science, surgery, coating, etc., can solve problems such as poor sustained release effect and affect the efficacy of peripheral drug-eluting stents, and achieve prevention of early burst release, prevention of restenosis, strong effect of drug action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
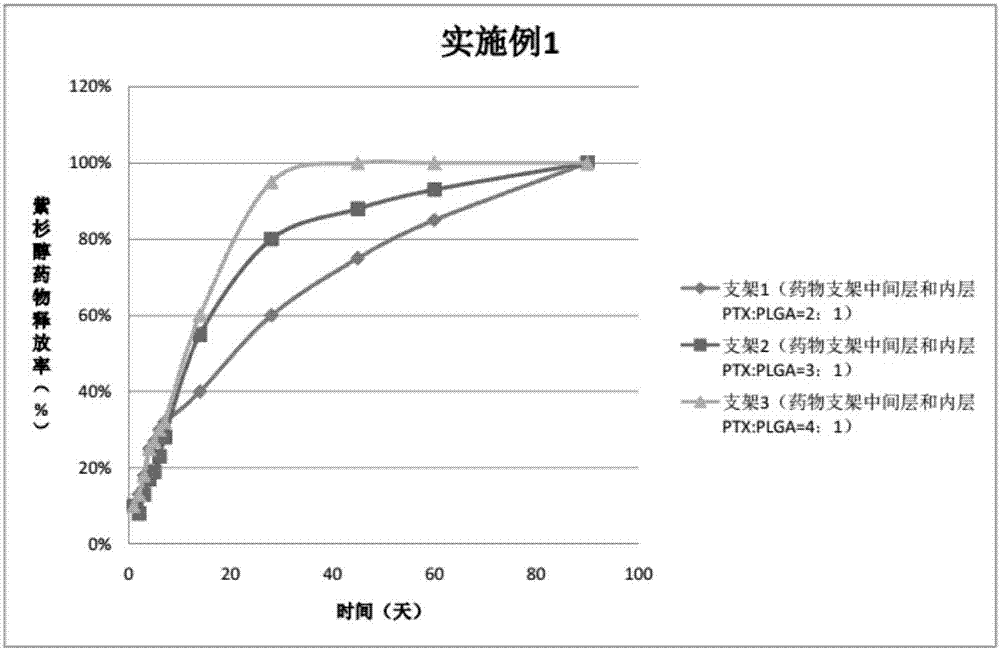
Embodiment 1
[0038] In the three peripheral drug-eluting stents provided in this embodiment, three drug coatings are sprayed on the outside of the three bare metal stents. The specific preparations of peripheral stents 1, 2, and 3 are as follows:
[0039] Preparation of spraying liquid for stent 1:
[0040] Drug configuration on the outer layer of the drug stent: Weigh 10mg PLGA (molecular weight 30000, PLA: PGA=70:30), add 10ml tetrahydrofuran, magnetically stir for 30-60min to completely dissolve the polymer;
[0041] Drug configuration in the middle layer of the drug stent: Weigh 20mgPTX and 10mgPLGA (molecular weight 20000, PLA:PGA=50:50), add 10ml tetrahydrofuran respectively, and magnetically stir for 30-60min to completely dissolve the polymer;
[0042] Drug configuration in the inner layer of the drug stent: Weigh 20 mg PTX and 10 mg PLGA (molecular weight: 20000, PLA: PGA = 80:20), add 10 ml of tetrahydrofuran, and magnetically stir for 30-60 minutes to completely dissolve the polymer.
[...
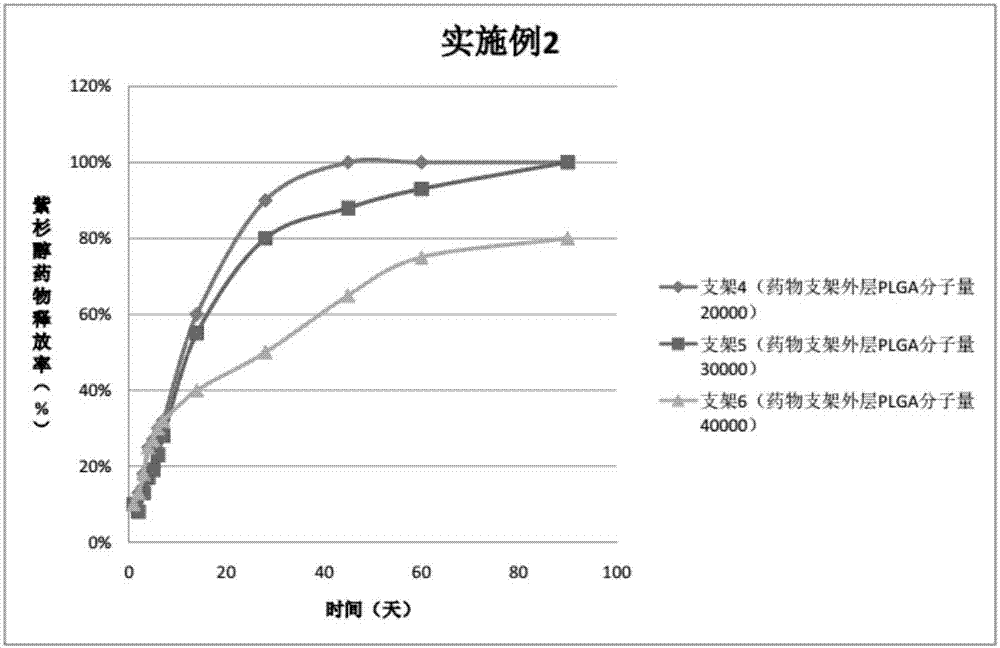
Embodiment 2
[0054] In the three peripheral drug-eluting stents provided in this embodiment, three drug coatings are sprayed on the outside of the three bare metal stents. The specific preparations of peripheral stents 4, 5, and 6 are as follows:
[0055] Preparation of spraying liquid for stent 4:
[0056] Drug configuration on the outer layer of the drug stent: Weigh 10mg PLGA (molecular weight 20000, PLA:PGA=70:30), add 10ml tetrahydrofuran, magnetically stir for 30-60min to completely dissolve the polymer;
[0057] Drug configuration in the middle layer of the drug stent: Weigh 30mgPTX and 10mgPLGA (molecular weight 20000, PLA:PGA=50:50), add 10ml tetrahydrofuran respectively, and magnetically stir for 30-60min to completely dissolve the polymer;
[0058] Drug configuration in the inner layer of the drug stent: Weigh 30 mg of PTX and 10 mg of PLGA (molecular weight: 20000, PLA: PGA = 80:20), add 10 ml of tetrahydrofuran, and magnetically stir for 30-60 minutes to completely dissolve the polyme...
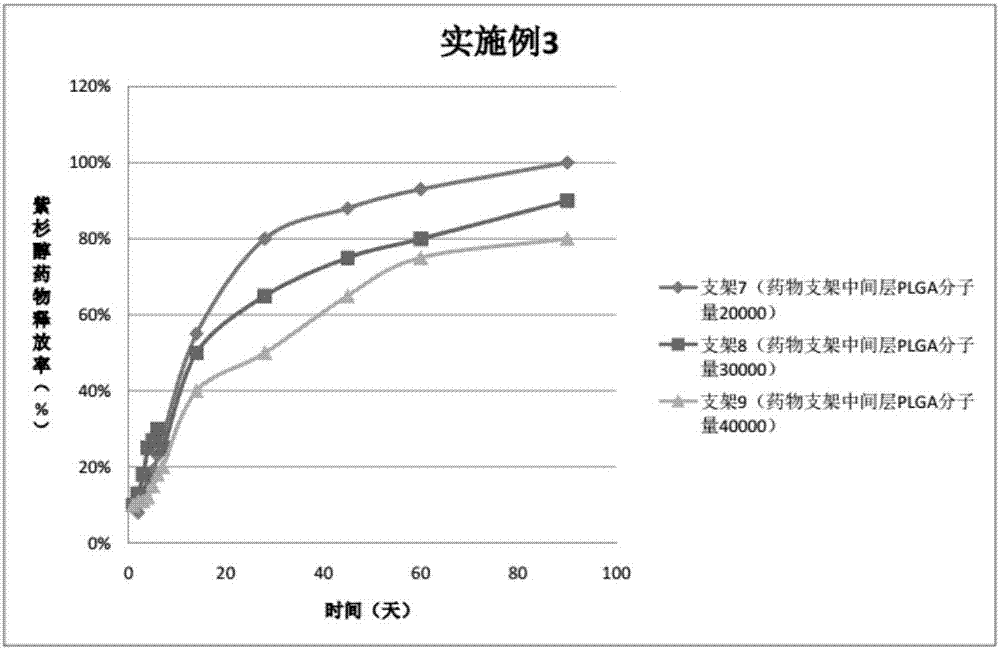
Embodiment 3
[0070] In the three peripheral drug-eluting stents provided in this embodiment, three drug coatings are sprayed on the outside of the three bare metal stents. The specific preparations of peripheral stents 7, 8, 9 are as follows:
[0071] Preparation of spraying liquid for stent 7:
[0072] Drug configuration on the outer layer of the drug stent: Weigh 10mg PLGA (molecular weight 30000, PLA: PGA=70:30), add 10ml tetrahydrofuran, magnetically stir for 30-60min to completely dissolve the polymer;
[0073] Drug configuration in the middle layer of the drug stent: Weigh 30mgPTX and 10mgPLGA (molecular weight 20000, PLA:PGA=50:50), add 10ml tetrahydrofuran respectively, and magnetically stir for 30-60min to completely dissolve the polymer;
[0074] Drug configuration in the inner layer of the drug stent: Weigh 30 mg of PTX and 10 mg of PLGA (molecular weight: 20000, PLA: PGA = 80:20), add 10 ml of tetrahydrofuran, and magnetically stir for 30-60 minutes to completely dissolve the polymer. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com