A kind of adjuvant composition and its preparation method and application
A technology of composition and adjuvant, applied in the field of adjuvant composition and preparation thereof, can solve the problems of no adjuvant effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of an adjuvant composition containing polyacrylic acid-ε-polylysine nanoparticles
[0035] Dissolve polyacrylic acid (commercially available Carbomer 934P) and polylysine in 0.85% NaCl solution, and adjust the pH thereof to 7.2 to obtain polyacrylic acid solution and polylysine solution. The polyacrylic acid solution and the polylysine solution are mixed under stirring conditions to obtain an adjuvant composition containing polyacrylic acid-ε-polylysine nanoparticles with negative charges on the surface.
[0036] The particle size of the formed nanoparticles was measured using DLS (Dynamic Light Scattering), showing that the formed nanoparticles had a particle size of 200-1500 nm. See Table 1 for the formulation of the adjuvant composition.
[0037] Table 1: Formulation Table of Adjuvant Compositions
[0038] group Carbomer 934P Polylysine Sodium chloride immune booster water 1 (Adjuvant 1) 0.5g 0.5g 0.85g — Add to ...
Embodiment 2
[0039] Embodiment 2: the promoting action of adjuvant composition of the present invention to porcine circovirus to produce neutralizing antibody
[0040] 2.1 Preparation of vaccine composition
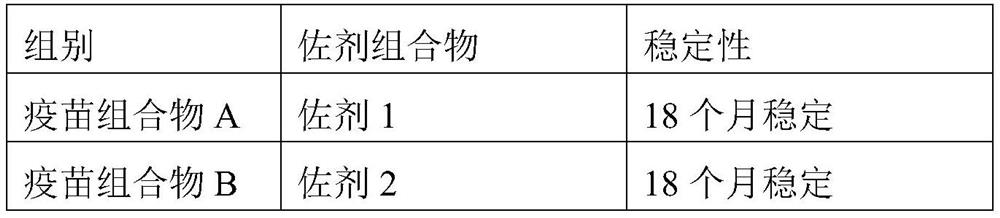
[0041] To 50 g of the adjuvant composition prepared in Example 1, 20 μg / head of PCV2 ORF2 protein was added, and the volume was constant with phosphate buffer at pH=7.4 to obtain a vaccine composition. The obtained vaccine composition was placed at 4°C to observe its stability, and the results are shown in Table 2.
[0042] Table 2: Formulation and Stability of Vaccine Compositions
[0043]
[0044]
[0045] It can be seen from Table 2 that the vaccine composition prepared by using the adjuvant composition of the present invention has high stability and can be kept stable for at least 18 months at 4°C.
[0046] 2.2 Neutralizing antibody titer experiment
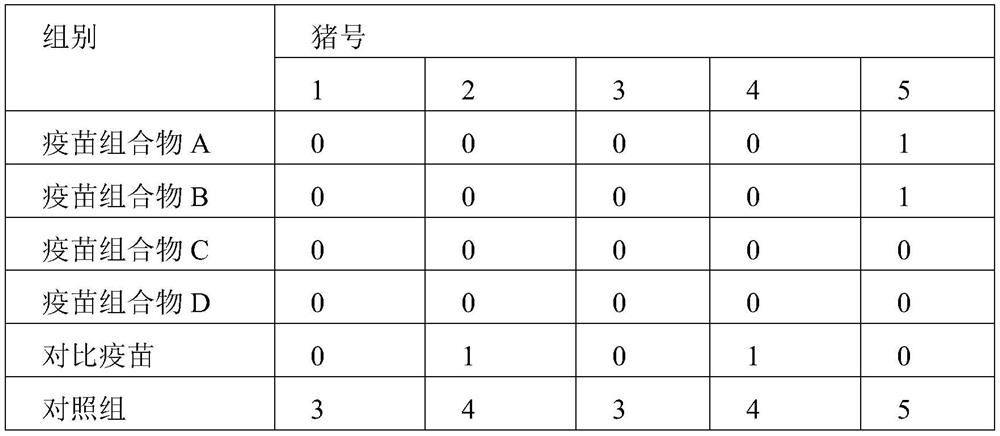
[0047] Thirty 30-day-old PCV2-negative healthy piglets were randomly divided into 6 groups with 5 piglets in each group. ...
Embodiment 3
[0067] Embodiment 3: The protection test of the adjuvant composition of the present invention to Mycoplasma hyopneumoniae
[0068] 3.1 Preparation of vaccine composition
[0069] In 50g of the adjuvant composition prepared in Example 1, add 2×10 8 / part of Mycoplasma antigen 50g to obtain the vaccine composition. The obtained vaccine composition was placed at 4°C to observe its stability, and the results are shown in Table 7.
[0070] Table 7: Formulation and Stability of Vaccine Compositions
[0071]
[0072]
[0073] 3.2 Test piglets immunized with vaccines
[0074] 30 piglets aged 14-21 days were selected and randomly divided into 6 groups, 5 piglets / group. The vaccine composition I, II, III, IV prepared in 3.1 and the comparison vaccine were respectively injected into the piglets of the 1st to 5th groups, and each piglet was injected with 1 ml of the corresponding inactivated vaccine in the neck muscle respectively; the 6th group was injected with the same amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com