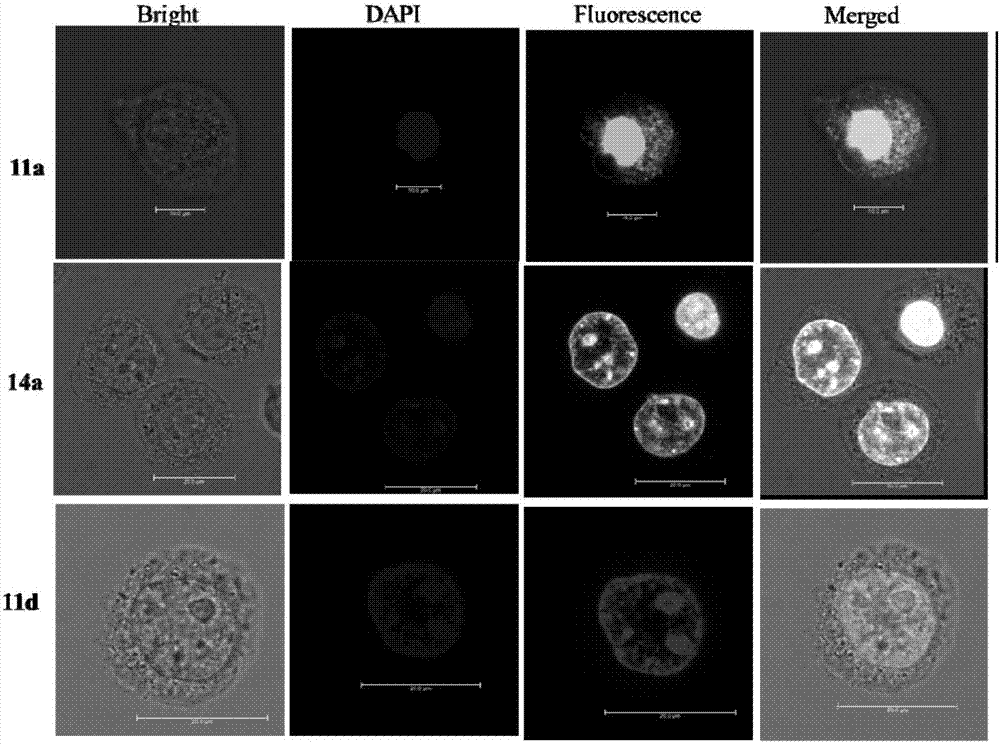
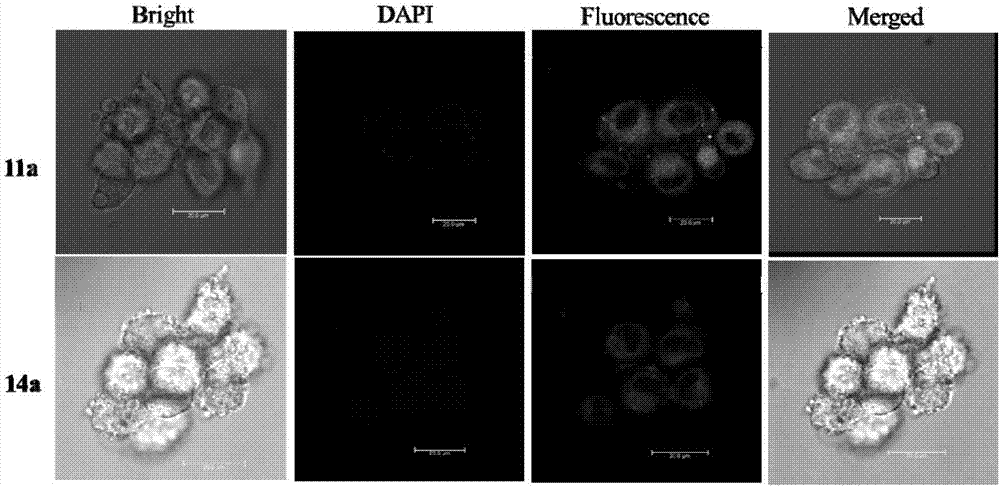
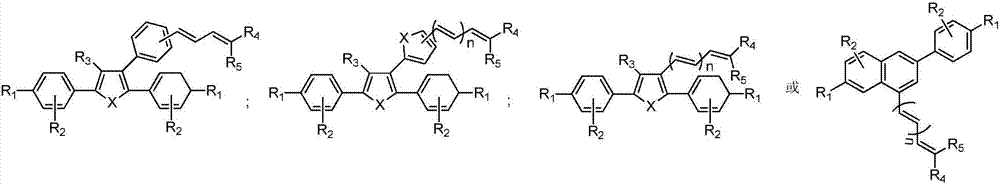
Targeted estrogen receptor fluorescent probes and preparation and usage methods thereof
An estrogen receptor and fluorescent probe technology, applied in the fields of medicine and biology, can solve the problems of side effects, the inability to dynamically detect the expression of estrogen receptors in real time, and the failure to use surgical navigation, and achieve a good fluorescence quantum yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of 3-bromo-2,5-bis(2-fluoro-4-methoxyphenyl)thiophene (1)
[0050] Weigh 2,3,5-tribromothiophene (0.32g, 1mmol) and p-methoxyphenylboronic acid (0.33g, 2.2mmol) in a 50mL round bottom flask, add tripotassium phosphate (0.64g, 3mmol), Tetrakis(triphenylphosphine)palladium (0.0058g, 0.005mmol), tetrabutylammonium bromide (0.032g, 0.1mmol), 5mL of dioxane, toluene and 5mL of water were reacted at 110°C for 24h, and the reaction was monitored by TLC After the reaction was complete, it was extracted three times with 50 mL of ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, filtered and spin-dried to obtain a crude product, which was purified by column chromatography to obtain a yellow solid product with a yield of 78%. 1 H NMR (400MHz, CDCl 3 )δ7.46(dt,J=12.9,8.9Hz,2H),7.32(s,1H),6.82-6.67(m,4H),3.85(s,3H),3.82(s,3H). 13 C NMR (101MHz, CDCl 3 )δ163.89(d, J=248.0Hz), 163.05(d, J=247.9Hz), 158.39(d, J=10.1Hz), 156.83(d, J=9.7H...
Embodiment 2
[0051] Example 2: Preparation of 3-bromo-2,5-bis(2-fluoro-4-methoxyphenyl)selenophene (2)
[0052] Referring to the preparation method of Compound 1, a yellow solid product was obtained with a yield of 81%. 1 H NMR (400MHz, CDCl 3 )δ7.51-7.28(m,3H),6.73-6.54(m,4H),3.73(s,3H),3.71(s,3H).
Embodiment 3
[0053] Example 3: Preparation of 3-bromo-2,5-bis(2-fluoro-4-hydroxyphenyl)thiophene (3)
[0054] Weigh compound 1 (0.41g, 1mmol) in a 50mL round bottom flask, add 5mL of dichloromethane, slowly add boron tribromide (0.75g, 3mmol) at 0°C, keep the reaction temperature for 8h, and monitor the reaction by TLC Completely, after the reaction was completed, ice water was added to quench the reaction, extracted three times with 50mL ethyl acetate, the organic layer was dried with anhydrous sodium sulfate, filtered and spin-dried to obtain the crude product, which was purified by column chromatography to obtain a white solid product with a yield of 88 %. 1 H NMR (400MHz, acetone-d 6 )δ9.24(s,2H),7.52(t,J=8.8Hz,1H),7.41-7.29(m,2H),6.88-6.67(m,4H). 13 C NMR (101MHz, Acetone) δ160.79(d, J=247.7Hz, 1H), 160.15(d, J=248.4Hz, 1H), 160.32(d, J=11.6Hz, 1H), 159.54(d, J =12.0Hz,1H),138.21(d,J=3.4Hz,1H),133.24(d,J=4.2Hz,1H),131.31(d,J=4.8Hz,1H),129.47(d,J=5.0 Hz,1H),127.86(d,J=5.4Hz,1H),112...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com